
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
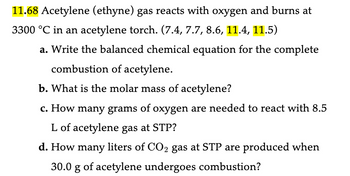
Transcribed Image Text:11.68 Acetylene (ethyne) gas reacts with oxygen and burns at
3300 °C in an acetylene torch. (7.4, 7.7, 8.6, 11.4, 11.5)
a. Write the balanced chemical equation for the complete
combustion of acetylene.
b. What is the molar mass of acetylene?
c. How many grams of oxygen are needed to react with 8.5
L of acetylene gas at STP?
d. How many liters of CO2 gas at STP are produced when
30.0 g of acetylene undergoes combustion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Similar questions
- Chloroform is a volatile (readily changes from liquid phase to gaseous phase) once commonly used in the laboratory but now being phased out due to its ozone depletion potential. If the pressure of gaseous chloroform in a flask is 195 Torr at 25°C and its mass for 1 liter of gas is 1.25 g, what is the molar mass (g/mol) of chloroform? O None of these O 119 g/mol 10.0 g/mol O 76.3 g/molarrow_forwarddetermine the density of a metal that has a mass of 2.17g and a volume of 0.35 cm^3arrow_forwardHow many mg in 1.35 g ? Report your answer to 3 decimal places (examples 1.042, 0.050, 0.198, 3.000).arrow_forward
- Ethyl mercaptan is an odorous substance added to natural gas to make leaks easily detectable. A sample of ethyl mercaptan weighing 3.17 mg contains 1.64 mg of sulfur. What is the mass percentage of sulfur in the substance?arrow_forwardA microorganism was cultured in a flask containing a liquid medium. Samples were taken hourly and number of cells counted using a counting chamber. The data are provided below in cells per mL. Draw a growth curve in Excel and use it to calculate generation time. The answer must be provided as a number (in minutes). Time Cells per mL 81235849 0 6 7 10 26 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 2.00E+04 2.20E+04 2.30E+04 2.40E+04 3.10E+04 5.00E+04 8.06E+04 1.30E+05 2.09E+05 3.38E+05 5.44E+05 8.78E+05 1.41E+06 2.28E+06 3.68E+06 5.93E+06 9.56E+06 1.54E+07 2.48E+07 4.01E+07 6.46E+07 1.04E+08 1.68E+08 2.71E+08 2.72E+08 2.73E+08arrow_forwardTell whether the entropy of the following reactions will be negative, positive or zero. b) NaNO3 + H2O -> Na^+ + NO3^- c) 4H + O2 <-> 2 H2O d) 4HO2 <-> 2H2O + O2 a) Solid Carbon Dioxide <-> Gas Carbon Dioxidearrow_forward
- A compound contains only nitrogen and oxygen. Analysis of a/an 8.234 g sample of the compound reveals that the compound contains 4.118 grams of oxygen. How many grams of nitrogen does the sample contain? I need help with the formula for this question.arrow_forward5arrow_forwardWhat is the Mass of 3.5 L of H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON