Question
thumb_up100%
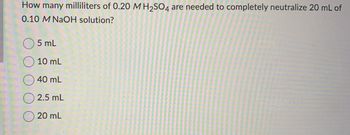
Transcribed Image Text:How many milliliters of 0.20 M H₂SO4 are needed to completely neutralize 20 mL of
0.10 M NaOH solution?
5 mL
10 mL
40 mL
2.5 mL
20 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For the reaction 2 H₂O → 2 H₂ + O2, how many moles of O2 are produced with 2.90 moles of H₂? Your answer should have 2 decimal places.arrow_forwardWhat is the half-life of the reaction when you begin with an initial concentration of AB= 0.80 M?arrow_forwardFour marbles are made of different metals. Each marble has the same mass, but a different volume. The density of each metal is given in the table. Metal Density (g/mL) scandium 2.99 silver 10.5 rhenium 20.8 tin 7.26 Place the marbles in order from largest to smallest. Largest tin marble scandium marble silver marble rhenium marble Smallestarrow_forward
arrow_back_ios
arrow_forward_ios