
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
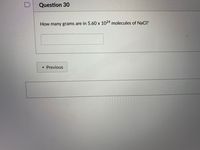
Transcribed Image Text:Question 30
How many grams are in 5.60 x 1024 molecules of NaCI?
Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Mercury is the only metal that exists as a liquid (d=13.56 g/cm3) at room temperature. Find the number of moles in 15 mL of mercury.arrow_forwardThe vapor pressures of the components, A and B, in a binary solution have been modeled and found to obey хара exp(0.75 XB) A A exp(0.75x) where XÃ and are the mole fractions, and PA* and PB* are the vapor pressures of each pure substance at room temperature. (a) If PA* = 0.084 bar and the total pressure of a mixture with XA = 0.40 is 0.125 bars, what is PB*, the vapor pressure of pure B (in bars)? P = X P А P₁ = X_P B QUESTION 14 B B * * Continuation of the previous problem (b) Assuming that the vapor is an ideal gas, what is the mole fraction of component B in the vapor phase?arrow_forwardTwo large tanks, each holding 100L of liquid, are interconnected by pipes, with the liquid flowing from tank A into tank B at a rate of 3L/min and from B into A at a rate of 1L/min. The liquid inside each tank is kept well stirred. A brine solution with a concentration of 0.1kg/L of salt flows into tank A at a rate of 6L/min. The (diluted) solution flows out of the system from tank A at 4L/min and from tank B at 2L/min. If initially, tank A contains pure water and tank B contains 10kg of salt, determine the mass of salt in each tank at time t≥0. What is the solution to the system? x(t)= y(t)=arrow_forward
- A young male adult takes in about 5.87 x 10-4 m3 of fresh air during a normal breath. Fresh air contains approximately 21% oxygen. Assuming that the pressure in the lungs is 1.09 x 105 Pa and air is an ideal gas at a temperature of 310 K, find the number of oxygen molecules in a normal breath.arrow_forwardCalculate the number of moles and atoms/molecules from the following: a. 67.83 grams of H2O moles _____________ Molecules _________________ b. 125.0grams of PCl3 moles _____________ Molecules_________________arrow_forwardIn a vacuum chamber designed for surface-science experiments, the pressure of residual gas is kept as low as possible so that surfaces can be kept clean. The coverage of a surface by a single monolayer requires about 10 19 atoms per m². What pressure would be needed to deposit less than one monolayer per hour from residual gas? You may assume that if a molecule hits the surface, it sticks.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON