
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
On the last page of the document I've attached a picture of the data collected. You can see five values (height of bubbles in mm) in each box. That is because my class was divided into five groups that each collected that data. The results become more valid when the data from multiple groups is averaged.
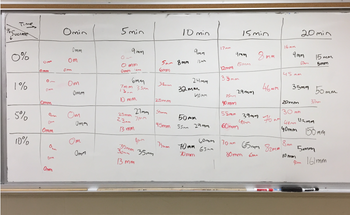
Transcribed Image Text:Time
%
90 Sucrose
0%
1%
5%
10%
0mm
0μm
Omm
Омм
Omm
0₂
Омм
Omm
Omin
0mm
Om
омм
Om
Omm
Om
Umm
От
Omm
5 min
9mm
0 mm
Отт 16 ми
6mm
7mm 3.5mm
13mm
10 mm
25mm
23mm
13 mm
qmm
35mm 35mm
30mm
13 mm
5mm 8mm
6mm
32mm
25mm
27mm 55mm
7mm
10 min
45mm
9mm
15mm
24mm
32mm
номи
50mm
55mm 29mm
75mm 70 mm
17mm
70 mm 65mm
70mm
9mm
15 min
15mm
12mm
35mm
29mm
36mm
40mm
55mm
60mm
8mm
46mm
39mm 76
47mm
KM
60mm 70 mm 65mm 82mm
80mm 67mm
16mm
9mm
20 min
45mm
17mm
15mm
5mm
35mm 50mm
10mm
20mm
30 mm
43mm
49mm
40mm 150mm
8mm
37mm
5mm
97m 161mm

Transcribed Image Text:SBI4U0
Fermentation in Yeast Lab
How does the concentration of sucrose affect the rate of fermentation of yeast?
Materials:
●
Warm tap water
Warm sucrose solutions: 1%, 5%,
and 10%
Baker's yeast (dry, active)
4 small balloons
4 large test tubes
Stopwatch
Procedure:
1.
Fill a large beaker halfway with tap water and place it on a hot plate. Create a
warm water bath at 38°C.
2. Stretch the balloons, and then blow them and release them several times to soften them and
increase their elasticity. Put them aside for step 6.
3.
Use a graduated cylinder to add 25 mL of warm tap water (at 38°C) to the first test tube, and
label it control.
4. Add 25 mL of each of the warm sucrose solutions (at 38°C) to the remaining three test tubes.
Label each test tube with the percentage of the sucrose solution.
5. Add 1.5 mL of yeast to each test tube.
6. Place a balloon over the top of each test tube. This will prevent any gases from escaping.
7. Carefully swirl or gently shake each test tube until the yeast dissolves.
8.
Place all four test tubes in the warm water bath that you set up in step 1.
9. Every 5 minutes for 20 minutes in total, measure the height of the bubbles and describe the
appearance of the reaction occurring and the appearance of the balloon for each test tube. (i.e.
measure at 0 minutes, 5 minutes, 10 minutes, 15 minutes, 20 minutes)
10. Record all observations in a table.
TO BE HANDED INTO DROPBOX:
Observations: (marked for completion - see below for requirements for graph)
■
■
●
Ruler
Graduated cylinder
Masking tape
Hot plate
Thermometer
Large Beaker
●
Plot a line graph (all data on one graph) to show the effect of sucrose concentration on amount of
fermentation over time.
o o o o o
Discussion Guiding Questions (marked for completion):
accurate
accurate axis titles with units
title - begins with "Figure 1:"
points plotted correctly and connected in line graphs
all sets of data on one graph with legend
organized logically and in a way to make easy comparisons
What was your independent variable? Dependent variable?
Why was yeast used? Why did the reaction need to be at 38 degrees?
What gas was causing the bubbles to form?
Explain the trends in the data.
How was sucrose (not glucose) used by the yeast cells for this process? (Hint: which enzyme(s) were
needed) → you may need to research this
Explain why at some sucrose concentrations the rate of fermentation may decrease after 15 minutes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- The homogeneity of the chloride level in a water sample from a lake was tested by analyzing portions drawn from the top and from near the bottom of the lake, with the following results in ppm Cl: Top Bottom 26.30 26.22 26.43 26.32 26.28 26.20 26.19 26.11 26.49 26.42 (a) Apply the t-test at the 95% confidence level to determine if the chloride level from the top of the lake is different from that at the bottom. At 95% confidence t crit = , and t = . Since t (>,<,=) we conclude that (a,no) significant difference exists at the 95% confidence level. (b) Now use the paired t-test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level. At 95% confidence t crit = and t = . Since t (>,<,=) we conclude that (a,no) significant difference exists at the 95% confidence level. (c) Why is a different conclusion drawn from using the paired t-test than from just pooling the data and using…arrow_forwardDichotomous Keys are used to identify an organism based on a choice between character states. Can you answer all the parts to this question please in complete sentences Based on the name, how many options should you have when working with a dichotomous key? Why does the term “dichotomous” let you know that? (hint: look up the Greek roots and meanings for this word)arrow_forwardIn spectrophotometry, we measure the concentration of an analyte by its absorbance of light. A low concentration sample was prepared, and nine replicate measurements gave absorbances of: 0.0047, 0.0054, 0.0062, 0.0060, 0.0046, 0.0056, 0.0052, 0.0044, 0.0058. Nine reagent blanks gave values of: 0.0006, 0.0012, 0.0022, 0.0005, 0.0016, 0.0008, 0.0017, 0.0010, and 0.0011. a) What is the absorbance detection limit? This is related to both the scatter of the blank and the scatter in the measured samples.arrow_forward
- If only the first five loci listed in Table 1 of the assigned paper (Forensics, DNA Fingerprinting, and CODIS) were analyzed, what would be the statistical probability of finding that particular genotype among people other than Suspect B? SHOW YOUR WORK! Hint: Multiply together the frequencies of the individual STR genotypes to obtain the overall profile frequency.arrow_forwardCan you help me to find answers thank you so much.arrow_forwardneed helparrow_forward
- Using the data below, calculate the correlation: covariance -0.9 standard deviation(x) = 10.5 standard deviation(y) = 10.4 (calculate to 2 significant figures)arrow_forward1) The interior of an axon at rest is negatively charged relative to the outside is positively charged relative to the outside has the same charge as the outside has the same concentration of potassium (K+) as the outside 2) The branch-like portions of a neuron that carries information toward the cell body is the axon. dendrite. terminal button. soma.arrow_forwardAfter graduating from UNC Charlotte with the BS in Biology, you are hired as a technician in a U. S. Department of Agriculture Research Station and assigned to a team that is investigating a new malady that has appeared in leaf cutter bees (shown below). Leaf cutter bees are solitary (i.e. non-social) bees which are essential pollinators for many of the crops that we feed livestock, such as alfalfa. The bees are becoming infected with a gram-positive bacteria that alters their immune response such that the bacteria cannot be eliminated and eventually the bees die. Your team is investigating how the bacteria might alter the bees’ immune response. You set up two groups of leaf cutter bees: the Treatment group is infected with the bacteria and the Control group is uninfected. You then compare the two groups for the different components of the insect immune system shown in the figures below (in Fig. 3 GLD = FAD-glucose dehydrogenase). Remember: P > 0.05 means the differences are…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education