
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How do you know that each element match up to their letter descriptions? I want a small explanation for each element if that is possible. Thank you.
| Alphabet | Element | Name | Alphabet | Element | Name | Alphabet | Element | Name |
| A | C | Carbon | J | Ca | Calcium | S | Ar | Argon |
| B | F | Flourine | K | Ne | Neon | T | Mg | Magnesium |
| C | I | Iodine | L | Be | Beryllium | U | Rb | Rubidium |
| D | Li | Lithium | M | K | Potassium | V | Ba | Barium |
| E | He | Helium | N | Ba | Barium | W | Cl | Chlorine |
| F | Fr | Francium | P | Kr | Krypton | X | Sn | Tin |
| G | Xe | Xenon | Q | Ge | Germanium | Y | Pb | Lead |
| I | Si | Silicon | R | Br | Bromine | Z | Na | Sodium |
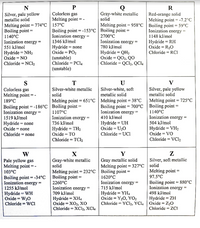
Transcribed Image Text:N
R
Colorless gas
Melting point
Gray-white metallic
solid
Red-orange solid
Melting point =-7.2°C
Melting point = 958°C | Boiling point = 59°C
Ionization cnergy =
Silver, pale yellow
metallic solid
Melting point = 774°C | 157°C
Boiling point
1140°C
Ionization energy =
Boiling point = -153°C | Boiling point
2700°C
Ionization energy =
Ionization energy =
1148 kJ/mol
Hydride = RH
Oxide = R2O
1346 kJ/mol
Hydride = none
Oxide = PO2
551 kJ/mol
780 kJ/mol
%3D
Hydride = NH2
Oxide = NO
Hydride = QH2
Охide %3D QO, QO
Chloride = QCI2, QC14
Chloride = RCI
(unstable)
Chloride = PC14
(unstable)
Chloride = NC12
S
Colorless gas
Melting point
189°C
U
Silver-white, soft
metallic solid
V
Silver, pale yellow
metallic solid
T
Silver-white metallic
solid
Melting point = 651°C | Melting point = 38°C
Melting point = 725°C
Boiling point = -186°C | Boiling point =
Ionization energy =
Boiling point = 700°C | Boiling point =
Ionization energy =
%3D
1140°C
1107°C
Ionization energy =
736 kJ/mol
1519 kJ/mol
410 kJ/mol
Ionization energy =
Hydride = UH
Oxide = U20
Chloride = UCI
504 kJ/mol
Hydride = none
Oxide = none
Hydride = TH2
Oxide = TO
Chloride = TCI2
Hydride = VH2
Oxide = VO
Chloride = none
Chloride = VC12
X
Y
W
Pale yellow gas
Melting point =-
103°C
Gray-white metallic
solid
Gray metallic solid
Melting point = 327°C
Melting point = 232°C | Boiling point =
Silver, soft metallic
solid
Melting point =
97.5°C
Boiling point = -34°C
Ionization energy =
1255 kJ/mol
Boiling point =
1620°C
Ionization cnergy =
%3D
Boiling point = 880°C
Ionization energy =
2260°C
%3D
Ionization energy =
715 kJ/mol
Hydride = YH,
Oxide = Y20, YO2
Chloride = YC12, YCI4
498 kJ/mol
Hydride = WH
Oxide = W20
Chloride = WCi
709 kJ/mol
Hydride = XH4
Оxide%3D XOz, хо
Chloride = XC12, XCI4
Hydride = ZH
Oxide = Z20
Chloride = ZCI
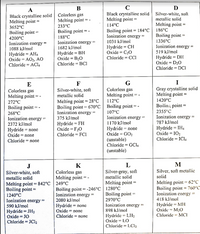
Transcribed Image Text:D
Silver-white, soft
mctallic solid
A
B
Black crystallinc solid
Melting point
Colorless gas
Melting point
233°C
Black crystalline solid
Melting point =
Melting point =
186°C
114°C
3652°C
Boiling point = 184°C
Ionization cnergy =
Boiling point
4200°C
Ionization energy =
1088 kJ/mol
Boiling point
188°C
Ionization cnergy =
1682 kJ/mol
Boiling point =
1031 kJ/mol
1336°C
Hydride = AH4
Oxide = AO2, AO
Chloride = ACI4
Hydride = BH
Oxide = B20
Chloride = BCI
Hydride = CH
Oxide = C20
Chloride = CCI
Ionization energy =
519 kJ/mol
Hydride = DH
Oxide = D20
Chloride = DCI
I
G
Colorless gas
Melting point =
112°C
E
F
Silver-white, soft
metallic solid
Gray crystalline solid
Melting point
Colorless gas
Melting point
1420°C
Melting point = 28°C
Boiling point = 670°C
Ionization energy =
272°C
Boiling point
Boiling point
Boilin; point =
2355°C
268°C
Ionization energy -
2372 kJ/mol
107°C
Ionization energy
1170 kJ/mol
375 kJ/mol
Ionization energy =
787 kJ/mol
Hydride = FH
Oxide = F20
Chloride = FCI
Hydride = none
Oxide = GO2
(unstable)
Chloride = GCI4
(unstable)
Нydride %3DIH4
Oxide = IO2
Chloride = ICl,
Hydride = none
Oxide = none
Chloride = none
J
K
M
Silver-gray, soft
metallic solid
Silver, soft metallic
solid
Colorless gas
Silver-white, soft
metallic solid
Melting point
= -
Melting point
Boiling point = -246°C | 1280°C
Boiling point
Melting point = 842°C | 249°C
Boiling point
1240°C
Ionization energy =
590 kJ/mol
Melting point -- 62°C
Boiling point = 760°C
Ionization energy =
418 kJ/mol
Hydride = MH
Oxide = M2O
Chloride = MCI
%3D
%3D
Ionization energy =
2970°C
Ionization energy =
2080 kJ/mol
Hydride = none
Oxide = none
Chloride = none
898 kJ/mol
Hydride = JH2
Oxide = JO
Chloride = JC12
Hydride = LH2
Oxide = LO
Chloride = LC12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please send me answer of this question immediately and i will give you like sure.send me typed answerarrow_forwardFill in the table. Element Number of Protons Number of Neutrons Number of Electrons Mass Number Charge 120 199 +2 Silicon 15 -1arrow_forwardIn 1911, Hans Geiger and Ernest Marsden, working under Ernest Rutherford, conducted an experiment involving alpha particles and gold foil that led to the discovery of the nucleus in atoms. The nucleus of an atom accounts for O most of the size and mass of the atom. O very little of the size and mass of the atom. O most of the atom's size but very little of its mass. O most of the atom's mass but very little of its size. 15 N 4 stv W MacBook Airarrow_forward
- Please find the attached image for answering the question.arrow_forward3. Which statement accurately describes the atoms of a specific element? A zinc, Zn, atom contains 30 protons inside the nucleus and 30 electrons outside the nucleus. An indium, In, atom contains 115 protons inside the nucleus and 49 neutrons outside the nucleus. A scandium, Sc, atom contains 45 electrons outside the nucleus and 21 neutrons inside the nucleus. An aluminum, Al, atom contains 27 electrons and 27 protons inside the nucleus. CLEAR ALLarrow_forwardplzz do all very easy , if u plan to do only 1 then skip plz do all in detail in neat and clean handwritingarrow_forward
- Name of Number of Number of electrons Number of SYMBOL A Charge element protons Neutrons zinc 30 34 17 -1 nitrogen 14 10 20arrow_forward8. Looking at the drawing below, which of the following is/are true of the region of the atom labeled “Y"? A. It contains electrons B. It contains very little mass compared to X C. It occupies a large space compared to X D. It is negatively charged E. It contains protons and neutronsarrow_forwardRosa's unknown made-up compound contains element D and element Z. It has a molar mass of 1070 g/mol Analysis of a sample shows that it contains 8.41% element D and 91.59% element Z What is its molecular formula of the made-up compound? Please refer to the Mini Periodic Table of Fake Elements to answer your questions Mini Periodic Table of Fake Elements O DZ2 O D20 Z11 D3Z7 O D6Z₁4 DJ 15.00 Avogadro's Number: 6.022 x 1023 19.00 Q Z 35.00 70.00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY