
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:V
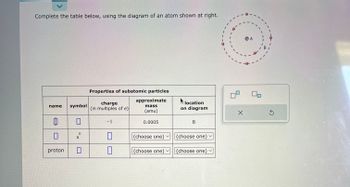
Complete the table below, using the diagram of an atom shown at right.
name
1
proton
symbol
0
0
n
0
Properties of subatomic particles
approximate
mass
(amu)
charge
(in multiples of e)
−1
10
0
0.0005
(choose one)
(choose one) ✓
location
on diagram
B
(choose one)
(choose one)
X
A
Ś
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the mass of Strontium-86 (in u) given that the average atomic mass of Sr is 87.6126 u and given the following dataarrow_forwardOnly 77 pleasearrow_forwardAn atom has a very small nucleus surrounded by an electron "cloud." The figure below represents the nucleus with a diameter of about 2 mm and describes the electron cloud as extending over 200 m. If the diameter of an atom is 1 x 10-° cm, what is the approximate diameter of its nucleus? Nucleus with protons (positive electric charge) and neutrons (no electric charge). Electrons (negative electric charge). The number of electrons and protons is equal in an electrically neutral atom. Diameter cmarrow_forward
- Enter your answer in the provided box. Calculate the atomic weight of the element given the mass and natural occurrence of each isotope. (Round to four significant figures.) Argon Mass (amu) Isotopic Abundance Argon−36 35.97 0.34% Argon−38 37.96 0.06% Argon−40 39.96 99.60% ___Amuarrow_forwardComplete the following table by filling in the missing information in each blank. Please only type in numbers Element Symbol Protons Neutrons Electrons Mass Number Mg 12 25 Xe 78 Fe3+ 30 BiS+ 83 209 68 68 167 (type in correct symbol for this one) Tch 米 f8 f12 10 fg 144 foarrow_forwardQuestion 2 The actual mass of a 17Cl atom is 36.9659 amu. Calculate the mass deficiency (amu/atom) for a 17 atom. Masses of subatomic particles: electron = .00055 amu; proton =1.0073 amu; neutron = 1.0087 amu Enter your three decimal places answer below. Round your answer to 3 decimal places.arrow_forward
- There are only two naturally-occuring stable isotopes of chlorine, the masses of which are listed in the table below. Use whatever data you need from the ALEKS Periodic Table to calculate the natural abundance of each isotope and complete the table. 35 Round your entry for C1 to 4 significant digits and your entry for isotope 35 Cl 37 Cl mass (amu) 34.969 36.966 natural abundance 1% % ☐x10 X 37 C1 to 4 significant digits. Śarrow_forwardPlease don't provide handwritten solution ....arrow_forwardUranium-238 undergoes radioactive decay until a stable isotope is reached. One of the steps is shown below. The chief would like to know what new elements are being produced. Determine the atomic mass, atomic number, and symbol of the new element based on the equation below. 4 227 Ac → ? + ½ ½ a >? 2 89 Atomic Mass Atomic Number Symbol Karrow_forward
- An aluminum atom has an average diameter of about 3.0 * 10- 8 cm. The nucleus has a diameter of about 2.0 * 10- 13 cm. Calculate the ratio of the atom’s diameter to its nucleus.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m3. If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.60 L .)arrow_forward[Atomic mass and isotopes] An unknown element has two naturally occurring isotopes: X- 69 and X-71. The mass of X-71 is 70.9163 amu, and its natural abundance is 49.31%. Calculate the mass and natural abundance of X-69. (Hint: The atomic mass of this unknown element is 69.90 amu.) Isotope mass (amu) natural abundance (%) X-69 X-71 70.9163 49.31arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY