
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For the isotopes, fill in the blank spaces.
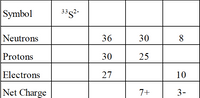
Transcribed Image Text:Symbol
33s2-
Neutrons
36
30
8
Protons
30
25
Electrons
27
10
Net Charge
7+
3-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An atom has two isotopes. the first isotope has a mass of 80.92 amu and a fractional abundance of 32.12%, and the second isotope has a mass of 78.23 amu. Find the atomic mass of this atom.arrow_forward100 Zinc has three major and two minor isotopes. For this problem, assume that the only isotopes of zinc are the major ones: zinc-64, zinc-66, and zinc-68. The atomic mass of zinc-64 is 63.9291 Da, the atomic mass of zinc-66 is 65.9260 Da, and the atomc mass of zinc-68 is 67.9248 Da. 57.4 Calculate the atomic mass of zinc from the relative peak intensities in the mass spectrum. 38.6 atomic mass: Da 64 66 68 mlz Peak intensityarrow_forwardAn unknown element is determined to have two naturally occurring isotopes. Isotope 1 is 68.11% abundant and has a mass of 68.925580 amu, and isotope 2 has a mass of 70.9247005 amu. Determine the atomic mass of the unknown element. Express your answer to four significant figures.arrow_forward
- The CRC Handbook, a reference book for chemistry and physics lists two isotope of Rubidium (Z-37). The atomic mass of 72.15 % of rubidium is 84.9118 u. The other isotope atomic mass was omitted (skipped) in typing. Calculate the atomic mass of the omitted isotope.arrow_forwardUse the dropdowns to create a correct and complete isotope notation. An isotope has 108 neutrons and 72 protons. The correct isotope is:arrow_forwardWhich of the following represents a pair of isotopes? Atomic Number | Mass Number 1. 6| 14 A II. 7| 14 1. 617 B II. 14 | 14 1. 6| 14 II. 14 | 28 I. 7 | 13 D II. 7 | 14 1. 8 10 II. 16 20arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY