
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
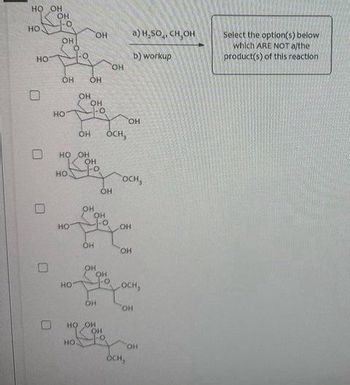
Transcribed Image Text:The image contains a problem related to a chemical reaction, possibly dealing with saccharide structures and methanol reagents. The task is to select the options that are not products of the given reaction.
**Reaction Description:**
- **Reagents:**
a) \( \text{H}_2\text{SO}_4, \text{ CH}_3\text{OH} \)
b) workup
**Task:**
Select the option(s) below which are NOT the product(s) of this reaction.
**Structures Presented:**
1. The reaction starts with a saccharide structure featuring multiple hydroxyl (OH) groups and is acted upon by the reagents.
2. Five structural options are given with checkbox selections alongside them.
- **First Structure:** A saccharide structure with three OH groups and three OCH₃ groups.
- **Second Structure:** A saccharide structure with four OH groups and two OCH₃ groups.
- **Third Structure:** A saccharide structure with two OH groups and four OCH₃ groups.
- **Fourth Structure:** A saccharide structure with three OH groups and three OCH₃ groups.
- **Fifth Structure:** A saccharide structure with one OH group and five OCH₃ groups.
These options represent different substitution configurations that result from the reaction conditions, involving methanol as the reagent. The task involves identifying which structures are not products of this specific chemical transformation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4N92 Crz O7+3C 6HIO +16H2 S04 >4Naz SOy + 4Crz(sO4)3 +3C6H10 04 1/6 H26 28,2009 of ColHio are reacted with excess Nazcr20, and H2 SO4.After the reaction is 118°8L Completed, 78.811grams of Crz (sou)3 are collected. Calculate precent yeild of the reachion * Sdve vin one Single Step Usinq dimensional analysısarrow_forwardConsider the calcination of CaCO3: CaCO3 -> CaQ + CO, Before calcination, the initial weight of CaCO3 is 2.5g. After calcination, the final weight of CaCO3 was measured at 2.0 g. Calculate the conversion of CaCO; particles (x) using the following equation: m CaCO, -m CaCo, ) x = m Caco, -MCao |arrow_forwardkindly answer 4-6. thank youarrow_forward
- Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HNO, (aq) + H,0(1) - Check Explanation O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility 80 F Cloudy DELL Delete PgUp Insert F12 F10 F11 F9 F7 F8 F5 F6 Hum F4 F2 F3 Loc Esc F1 Backspace & Fn # 7 8 5 %24arrow_forwardChoose the coefficients that balance the following reaction: C3H5(NO3)3 → CO2 + N2 + H20 + 2:6:3:3:1 O 4:12:6:10:1 O 1:1:1:1:1:1 2:1:2:1:2 %23 %24 & backspace 21 3. 9. e r y tab a f k C V cularrow_forwardCTQ 10: Calculate the AHrxn for the reaction of C(diamond) and 0,(g) to form Co,(g) using the reactions in Model 3 by adding, reversing, or multiplying the reactions given below as needed. C(graphite) C(diamond) = 1.9 kJ/mole AH xn C(graphite) + 0,(g) → CO,(g) AHxn = -393.5 kJ/molel Answer herearrow_forward
- How do we calculate the yield -- based on the following: Isopentyl alcohol (MW=88.2; d=0.813). Isopentyl acetate (MW=130.19; d=0.9 g/mL). Any assistance and breakdown of steps would be helpful. thanks!arrow_forwardPredict the products of the resulting reactions - all will occur. 1) combination rxn: Na + Cl, --> 2) decomposition: PbO --> 3) (single or displacement, Li is more active) Li + CoN --> 4) (double or exchange) Ca(NO3), + NaOH --> o search W Cop 果 f10 144 A % & * L. 8. Uarrow_forwardContent MasteringChemistry: PS 10-CI X session.masteringchemistry.com/myct/itemView?assignment ProblemID=206802692&attemptNo=1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY