
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
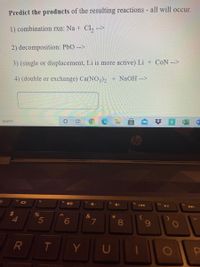
Transcribed Image Text:Predict the products of the resulting reactions - all will occur.
1) combination rxn: Na + Cl, -->
2) decomposition: PbO -->
3) (single or displacement, Li is more active) Li + CoN -->
4) (double or exchange) Ca(NO3), + NaOH -->
o search
W
Cop
果
f10
144
A
%
&
*
L.
8.
U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- October 3rd 2022 Question 56: Working backwards, deduce the starting material that led to the indicated product through the defined reactions. (a) A (b) B (1) HCOCI, AICI, (2) CI₂, AICI, (3) NaBH, MeOH (4) NaH, Etl (1) CH,CI, AICI, (2) HNO₂, H₂SO (3) KMnO, HO", A (4) LIAIH, in Et,O (5) H₂O/H₂SO CI CI HO OEt on It NO₂arrow_forwardme 1- Ed D File 1012 66 30 Edit View ← → C History Bookmarks F2 Aktiv Chemist X app.101edu.co Lectures Mahreen Ahmed 2d 10 Profiles Tab F3 3.1 Electroma X 2 Aluminum nitrate forms a white crystalline hydrate with the formula AI(NO3)3.nH₂O(s). If this hydrate is heated to a high enough temperature, H₂O(g) can be driven off, leaving the anhydrous salt AI(NO₂),(s). A 15.152 gram sample of the hydrate was heated to drive off the water, leaving Al(NO)(s) with a mass of 8.603 grams. Calculate the value of n in Al(NO)¸·nH₂O(s). SEP 11 Window Help F4 = 6.2 Determ Desmos | Sci x F5 Question 27 of 46 tv ♫ @ F6 2 MacBook Air 2 K F7 Feet to Meter x formula for v x C 1 DII FB 4 7 +/- all Z A 2 2 5 8 F9 | Carrow_forwardCHEMICAL REACTIONS Soad V Balancing chemical equations with interferin... CH, CH, (g) + 0,(g) - co,(g) + H,0(g) ロ→ロ olo Ararrow_forward
- Explain Tamoxifen reaction step by step and tell which compounds are reactants, which compounds are products and which compounds are solventarrow_forwardFrom the reaction CaCO3+CO2+H2O= Ca2+ + 2HCO3- A.) Show how it affects the chemistry of water, alkalinity and hardness of water B.) Its relationship to microbial degradation of organic matterarrow_forwardComplete the balanced molecular chemicahal equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. RB2CO(aq)+Fe(C2H3)2)2(aq)> Please explain Thanks.arrow_forward
- What is the mechanism by which the following reaction occurs ?: [Ru(NH3)6]2+ + [Fe(H2O)6]3+ → [Ru(NH3)6]3+ + [Fe(H2O)]2+ Select one: a. Dissociative mechanismb. Inner sphere mechanismc. External sphere mechanismd. Associative mechanismarrow_forwardPbF2 has a Ksp of 3.7 x 10-8. If you have a solution of PbF2 that has a fluoride ion concentration equal to 4.0 x 10-3, the solution is ... (Assume that the only relevant reaction is the solubility-product equilibrium.)arrow_forwardGiven the reaction that I added. How many grams of [Ni(en)3]SO4 should you use in order to make 1.25g of PbSO4? How many milliliters of 0.10M Pb(NO3)2 should be used to make this reagent in 10% mole excess?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY