
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
![### Question
What mass (in grams) of aspirin (\(C_9H_8O_4\)) is produced from 67.3 g of \(C_7H_6O_3\) assuming 95.0% yield from the reaction below?
\[
C_7H_6O_3 (s) + C_4H_6O_3 (l) \rightarrow C_9H_8O_4 (s) + HC_2H_3O_2 (aq)
\]
### Explanation
In this question, we need to calculate the theoretical and actual mass of aspirin produced from the given starting material, \(C_7H_6O_3\) (salicylic acid), assuming a reaction yield of 95.0%.
### Steps to Solve
1. **Balanced Chemical Equation**:
\[
C_7H_6O_3 (s) + C_4H_6O_3 (l) \rightarrow C_9H_8O_4 (s) + HC_2H_3O_2 (aq)
\]
Here, one mole of \(C_7H_6O_3\) reacts with one mole of \(C_4H_6O_3\) to produce one mole of \(C_9H_8O_4\) (aspirin) and one mole of acetate.
2. **Molar Mass Calculation**:
- Molar mass of \(C_7H_6O_3\) (salicylic acid) = 138.12 g/mol
- Molar mass of \(C_9H_8O_4\) (aspirin) = 180.16 g/mol
3. **Moles of Salicylic Acid**:
\[
\text{Moles of } C_7H_6O_3 = \frac{67.3 \, \text{g}}{138.12 \, \text{g/mol}} \approx 0.487 \text{ mol}
\]
4. **Theoretical Yield of Aspirin**:
\[
\text{Theoretical moles of } C_9H_8O_4 = \text{Moles of } C_7H_6O_3 = 0.487 \](https://content.bartleby.com/qna-images/question/38543b38-0ca2-4898-a226-639f5edfcbc9/36f76b53-4996-48e7-b038-c710f4bbb855/rgfwy3_thumbnail.jpeg)
Transcribed Image Text:### Question
What mass (in grams) of aspirin (\(C_9H_8O_4\)) is produced from 67.3 g of \(C_7H_6O_3\) assuming 95.0% yield from the reaction below?
\[
C_7H_6O_3 (s) + C_4H_6O_3 (l) \rightarrow C_9H_8O_4 (s) + HC_2H_3O_2 (aq)
\]
### Explanation
In this question, we need to calculate the theoretical and actual mass of aspirin produced from the given starting material, \(C_7H_6O_3\) (salicylic acid), assuming a reaction yield of 95.0%.
### Steps to Solve
1. **Balanced Chemical Equation**:
\[
C_7H_6O_3 (s) + C_4H_6O_3 (l) \rightarrow C_9H_8O_4 (s) + HC_2H_3O_2 (aq)
\]
Here, one mole of \(C_7H_6O_3\) reacts with one mole of \(C_4H_6O_3\) to produce one mole of \(C_9H_8O_4\) (aspirin) and one mole of acetate.
2. **Molar Mass Calculation**:
- Molar mass of \(C_7H_6O_3\) (salicylic acid) = 138.12 g/mol
- Molar mass of \(C_9H_8O_4\) (aspirin) = 180.16 g/mol
3. **Moles of Salicylic Acid**:
\[
\text{Moles of } C_7H_6O_3 = \frac{67.3 \, \text{g}}{138.12 \, \text{g/mol}} \approx 0.487 \text{ mol}
\]
4. **Theoretical Yield of Aspirin**:
\[
\text{Theoretical moles of } C_9H_8O_4 = \text{Moles of } C_7H_6O_3 = 0.487 \
Expert Solution
arrow_forward
Step 1
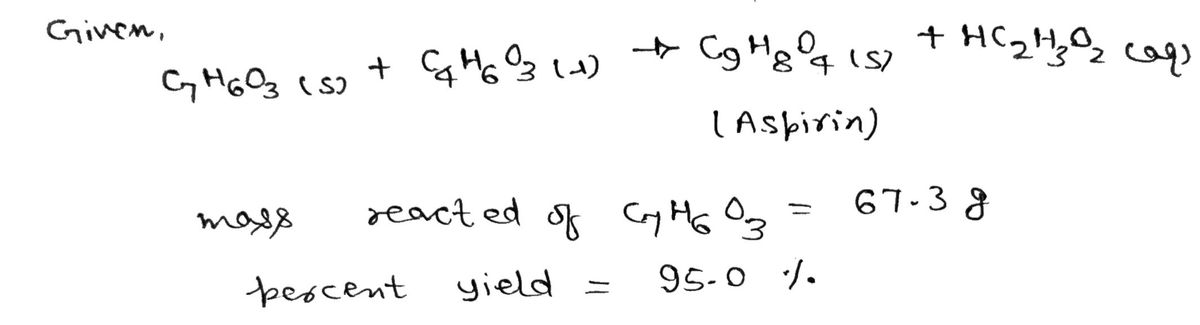
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. What mass of oxygen is produced if 22.7 mol of carbon dioxide is consumed in a controlled photosynthesis reaction? 6CO₂(g) + 6H₂O(l) → C6H12O6(s) + 60₂(g)arrow_forward2.) Five standardized dilutions of aqueous lead(II) chromate solution were prepared by adding a volume of 0.0010 M PbCrOa(ag) from a buret and then diluting the solution with deionized water to a total volume of 100.0 mL. Use the provided information to determine [CrO42] in each. Hint: enter the decimal before the scientific notation. Mixture Initial Buret Reading (mL) Final Buret Reading (mL) [CrO4?] (M) 5 16.83 42.60 ? x 104arrow_forwardWhat mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forward
- Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+Cl2(g) How much MnO2(s) should be added to excess HCl(aq) to obtain 285 mL Cl2(g) at 25 °C and 95 kPa?arrow_forwardWrite and balance the reaction for the complete combustion of nonyne, C9H16.arrow_forwardCryolite (Na3AlF6) is used in the commercial production of aluminum from ore. Cryolite itself is produced by the following reaction: 6 NaOH + Al₂O3 + 12 HF → 2 Na3AlF6 + 9 H₂O A mixture containing 430.0 kg of NaOH, 232.7 kg of Al2O3, and 600.0 kg of HF is heated to 950 °C until it reacts to completion. What is the maximum mass of Na3AlF6 formed? kg Na3AlF6arrow_forward
- what is the percent yield of the reaction if 2.00 grams of sodium benzoate are protonated with an excess of 3M aq. hydrochloric acid to produce 1.55 grams of benzoic acid?arrow_forward21. Nitric acid is manufactured in the Oswald process, which involves the catalytic oxidation of ammonia according to the following equations: (i) 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g) (ii) 2 NO(g) + O2(8) 2 NO2(g); (iii) 3 NO2(g) + H2O(1) → 2 HNO3(aq) + NO(g); (NO is fed back into the second reactor) (a) The above equations may be combined to yield the following equation. Balance this equation. NH3(g) +_O2(g) + _H2O(1) _HNO3(1) + _H,O(1) + NO(g) (b) What is the mole fraction of NH3 that becomes nitric acid in one reaction cycle? (c) How many kilograms of HNO3 will be produced in one reaction cycle from 1.00 m³ of NH3, measured at STP, if the overall yield is 92.0%? (d) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has density of 1.48 g/mL, how many litters of concentrated nitric acid is produced in this process at the above reaction yield? (Answers: (b) 2/3; (c) 1.73 kg of HNO3; (d) 1.67 L)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY