
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
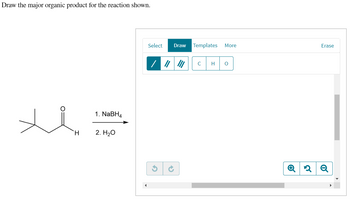
Transcribed Image Text:**Instruction:**
Draw the major organic product for the reaction shown.
**Chemical Reaction:**
The reaction starts with an aldehyde, represented by the molecular structure on the left. The structural formula consists of a carbonyl group (C=O) with a hydrogen atom bonded to one side and an isopropyl group (a branched carbon chain) on the other side.
**Reagents:**
1. NaBH₄ (Sodium borohydride)
2. H₂O (Water)
**Procedure:**
1. Sodium borohydride (NaBH₄) is used as a reducing agent to convert the aldehyde into the corresponding alcohol by adding hydrogen to the carbonyl group.
2. The reaction is then quenched with water, facilitating the completion of the reduction to form the alcohol.
**Diagram:**
On the right side, an editing interface is displayed with options to draw, select, and erase chemical structures. The icons and buttons allow for manipulation of chemical drawings, indicating that the user needs to sketch the expected alcohol product acknowledging the reduction process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. The synthesis of aspirin (acetylsalicylic acid) is shown below. ملا OH OH salicylic acid ("SA”) ● + H3C CH3 acetic anhydride ("AA") H3C OH aspirin ("AS") a) If you start with 0.25 grams of salicylic acid and 2.0 mL of acetic anhydride (d=1.08 g/mL), how much aspirin can you theoretically synthesize? + CH3COOH Show two calculations for the theoretical yield of aspririn: 1 with 0.25 g SA and 1 with 2.0 mL of AA. Compare the theoretical amounts of aspirin from both calculations to determine the limiting reagent. Circle/label the limiting reagent and its theoretical yield of aspirinarrow_forwardCalcium phosphate (Ca3(PO4)2) is a chemical that can be added during water treatment and the chemical undergoes the following reaction when added to water. Ca3(PO4)23 Ca²+ + 2 PO³- If 37 grams of 94% pure Ca3(PO4)2 is added to 55 liters (L) of water, find the calcium (Ca²+) concentration in units of milli-moles per liter (mM). Report your answer to the nearest hundredths (0.01) place.arrow_forwardDetermine the mol H2O/mol CaSO4 if you have the following data to two decimal places (MM H2O =18.02 g/mol; MM CaSO4 = 136.14 g/mol) mass of sample 1 mass of water in sample 0.23 mass of CaSO4 in sample 0.76arrow_forward
- Ex. 110 - Calculating Mass of 3 attempts left Check my work What mass of oxygen is needed to react with 1.79 gal of methanol according to the balanced equation below? (1.00 gal = 3.79 L, and the density of methanol is 0.793 g/mL.) 2 CH3OH() + 3 02g) – 2 CO2g) + 4 H2O(g) If appropriate, express your answer in scientific notion. (Click on the answer box to show the pallet.) < Prev 3 of 5 ***** ****arrow_forward27 A chemist prepares a solution of sodium chloride (NaCl) by measuring out 0.50 g of NaCl into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl anions in the chemist's solution. do Be sure your answer is rounded to 2 significant digits. 18 Ar mol x10 L Submit Assignment Continue 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center Accessibility Show All IMG-6095.jpg IMG-6096.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DD DII F12 F11 F10 F9 80 F7 F8 F6 F5 F4 esc F2 F3 F1 & % @ # 7 8 3 4 5 6 1 2 { P E R Y Q W %24arrow_forwardOzone (O3) decomposes into molecular oxygen according to the equation below. 2 O3 (g) --> 3 O2 (g) If you had a sample of ozone which decomposes into oxygen, which of the following statements is true? a. The mass of the oxygen at the end is greater than the mass of the starting ozone. b. The volume of the oxygen at the end is less than the volume of the starting ozone. c. The total number of molecules does not change during the reaction. d. The total number of atoms does not change during the reaction.arrow_forward
- A chemist adds 1.70 L of a 0.39 mol/L barium chloride (BaCl) solution to a reaction flask. Calculate the millimoles of barium chloride the chemist has added to the flask. Round your answer to 2 significant digits. manol O.P Xarrow_forwardWhat is the molar volume of solid quartz, SiO2?(density =2.65g/cm3) (molar volume is the volume occupied by one mole)arrow_forward4. A student conducted an experiment by heating BaCl₂ 2H₂O and reported the percent of water as 15.25%. Calculate the percent error (Hint: Use the calculated theoretical value).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY