
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
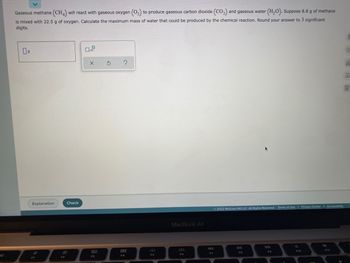
Transcribed Image Text:Gaseous methane (CH4) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 8.8 g of methane
is mixed with 22.5 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant
digits.
D
X
3
?
B
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
P
DII
F10
Explanation
Check
38²
80
888
F4
FS
MacBook Air
Fo
J
ol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 4-M sample of hydrochloric acid, HCl, has a density of 1.42 g/ml. What is the mass % (wt/wt) of the sample? Use the following atomic masses: H = 1, Cl = 35.45. Express your answer in percentage and in two decimal places.arrow_forwardA student is trying to figure out how many moles of potassium chloride are made from 4.0 grams of chlorine gas. Which step in the student's work below is incorrect? 2 K+ Cl →2 KCı 1 moles Cl2 1 moles KCl 2 moles Cl, 4.0 g Cl2 X 1 70.915 g Cl2 Step 1 Step 2 Step 3 Step 3 Step 2 There is no mistake. Step 1 O 00Oarrow_forwardHow many sodium ions are present in 20.0 mL of 0.10 M Na2Sarrow_forward
- Balance the following chemical reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. iron (II) sulfide + oxygen gas → iron (III) oxide + sulfur dioxidearrow_forwardThe formula to use for calculations of dilutions is 1. VịC = V½C2 %3D 2. VịC2 = V½C1 V2 3. a %3D 4. O 1 O 2 O 3 O 4 ||arrow_forwardortin X TETAAZEN ce/acellus_engine.html?ClassID=177151 tions + Use the data below to determine the mass ratio of iron to oxygen for compound 1. Et Compound 1 2 nternational Academy of Science. All Rights Reserved. Mass Fe (grams) 15.55 20.98 [P]:1 Enter the answer that belongs in the green box. Iron Ratio Cmpd 1 Mass O (grams) 4.45 9.02 * C Enterarrow_forward
- How many grams of lithium nitrate will be needed to make 200.0 grams of lithium sulfate, assuming that you have an adequate amount of lead (IV) sulfate to do the reaction? How much lead (IV) sulfate will you actually need? Pb(SO4)2 + 4 LiNO3 --> Pb(NO3)4 + 2 Li2SO4 Make sure you have the right number of significant figures.arrow_forwardand (II) chloride. are formed when aluminum reacts with cobalt If no reaction occurs, type NR in both boxes. Please use symbols, not full names, in your answer. For subscripts, please use full-size numbers. For example, H₂O would be entered as H20. Please don't confuse the letter "o" with the number "zero". Please don't confuse the letter "i" with the letter "1". and The products are Enter the free metal first. Enter the ionic compound second. Please include (aq), (I), (s) or (g) in your answers.arrow_forwardYou have a lot of paperwork laying around. A ream of stacked paper is 500 sheets and is 2.0 inches thick. Let's say you had a mole of paper. (1 mole of paper = 6.022 x 10^23 sheets of paper) and it was all stacked up in 10 stacks of equal height, each containing a tenth of a mole of sheets. The ten stacks sit one on top of the other. How high are they?arrow_forward
- Calculate the number of moles of hydrogen gas produced. Round your answer to 2 significant digits.arrow_forward[Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forwardIs H₂O₂ being created or destroyed by the chemical reaction? If H₂O2 is being created or destroyed, what is the rate at which it is being created or destroyed 1000 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If H₂O₂ is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 1000 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. ооо created destroyed neither created nor destroyed 0 x10 00 X Ś 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY