
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
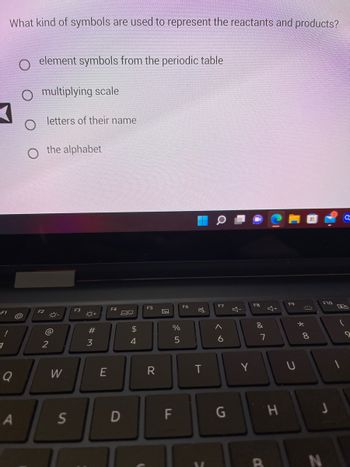
Transcribed Image Text:**Question: What kind of symbols are used to represent the reactants and products?**
1. Element symbols from the periodic table
2. Multiplying scale
3. Letters of their name
4. The alphabet
*Note: The first option, "Element symbols from the periodic table," is selected.*
Expert Solution
arrow_forward
Step 1
Any reactant and product are represented by chemical formula which is written using Elemental symbols.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer question 18 Part A and Barrow_forwardIron is found in Earth's crust as several iron compounds. Calculate the mass in kilograms of the amount of each of the following iron compounds that contains 1.0×103kg1.0×103kg of iron. Fe2O3Fe2O3 (hematite) Fe3O4Fe3O4 (magnetite) FeCO3FeCO3 (siderite)arrow_forwardA molecular compound has the following empirical formula: CH2O. The molar mass of the empirical formula is __________ g. Write your answer using 3 significant figures. If the molar mass of the molecular compound is 180.0 g/mol, write the molecular formula of the compound. ______________arrow_forward
- Determine the formula weight of caffeine, C₈H₁₀N₄O₂. Provide an answer to two decimal places.arrow_forwardFill in the Blanks Chemical Price/kg (in dollars) NaOH 21.17 CaCl2 41.28 КОН 31.38 CUSO4 93.32 NH4NO3 59.64 NH4CI 25.04 NaCl 21.15 NaNO3 72.35 Type your answers in all of the blanks and submit Another requirement is that the cost of the salt needs to be evaluated to determine if it is economical to use. To do this, you should consider the price per degree of dropping the temperature of the solution. The table of prices per kg for each salt is shown above. Let's determine the cost for the NaOH solid as an example. We want to determine how much it costs to change the temperature by 1 deg C so we start with the cost given in the table and end with $/deg C. For NaOH: 21.17 $/ 1 kg) X ( 1 kg/ 1000 X ( Type your answer here g/ Type your answer here deg C) Please type your answer to submit Please type your answer to submit Type your answer here $/deg Carrow_forwardA student calculated the number of moles of carbon dioxide present in a 100.0g of gaseous mixture. If the student determined that there is 0.30 mole of carbon dioxide present in the same, what is the percent of carbon dioxide in the sample?arrow_forward
- Which of the following best describes the system depicted in the diagram? Mixture of elements Element Compound Mixture of compoundsarrow_forwardStudents react zinc with hydrochloric acid to produce zinc chloride and hydrogen gas in a test tube. They weighed the reactants, the flask, and the balloon that captured the hydrogen gas. The zinc was added to the acid and the balloon attached across the mouth of the flask. The table shows the data each group collected. Zinc Zinc & Chloride Hydrochloric & |Group acid Hydrogen gas 155.67 g 155.65 g 2 157.80 g 157.78 g 3 148.22 g 146.54 g 4 155.93 g 155.92 g 169.42 g 169.43 g Explain how the data supports the Law of Conservation of Mass is followed.arrow_forwardWhich of the following is a chemical property? A length of a matchstick B color of a matchstick C ability of a matchstick to float on water D ability of a matchstick to burn when struckarrow_forward
- Physical Properties Malleability (Malleable means- (it shatters or can be hammered into thin sheets) Brittle Physical Characteristics (color, luster, flaky, powder, etc.) Element Symbo cracks when struck) 1 Yes/No Yes/No 1. Aluminum 2. Carbon 3. Iron 4. Magnesium 5. Silicon 6. Sulfur 7. Tin 8. Zincarrow_forwardDetermine the mol H2O/mol CaSO4 if you have the following data to two decimal places (MM H2O =18.02 g/mol; MM CaSO4 = 136.14 g/mol) mass of sample 1 mass of water in sample 0.23 mass of CaSO4 in sample 0.76arrow_forwardFigure 1 Figure 2 H₂O2 (1)- ---> H₂O2 (g) H₂O₂(aq) ---> H₂O (I) + O₂ (g) Which of the following statements BEST explain which figure is a model of a chemical change? O Figure 2, because there are 3 states of matter present. Figure 1, because H₂O2 changes state of matter. Figure 2, because new substances are produced. Figure 1, because H₂O2 created a product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY