
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
Show work. don't give Ai generated solution
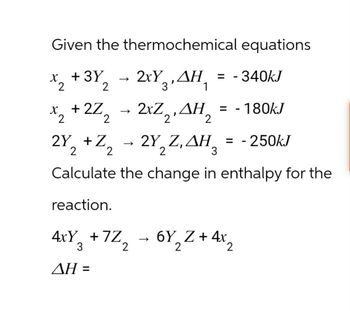
Transcribed Image Text:Given the thermochemical equations
x2 +32 2xYAH = -340kJ
->
->
ΔΗ
*2+2Z2 - 2xZ2,AH2 = -180kJ
2Y2 + Z2
-2Y, Z, AH,
H = -250kJ
2
Calculate the change in enthalpy for the
reaction.
3
4xY₂ +7Z
+7Z2
->
6Y2Z+4x2
AH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Would the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or remain the same if the heat capacity of the calorimeter were taken into account? Explain your answer.arrow_forwardWhen one mol of KOH is neutralized by sulfuric acid, q=56 kJ. (This is called the heat of neutralization.) At 23.7C, 25.0 mL of 0.475 M H2SO4 is neutralized by 0.613 M KOH in a coffee-cup calorimeter. Assume that the specific heat of all solutions is 4.18J/gC, that the density of all solutions is 1.00 g/mL, and that volumes are additive. (a) How many mL of KOH is required to neutralize H2SO4? (b) What is the final temperature of the solution?arrow_forwardIf the heat capacity varies withtemperature, abetter form ofequation 2.9 isto solve q=TiTfnCT-t A 50.0-g sample of white phosphorus is heated from 298 K to 350K. If its molar heat capacity is CT- = 56.990.1202T J/mol.K, how much heat is needed?arrow_forward
- Benzoic acid, C6H5COOH, is a common standard used in bomb calorimeters, which maintain a constant volume. If 1.20 g of benzoic acid gives off 31, 723 J of energy when burned in the presence of excess oxygen and in a water bath having a temperature of 24.6 C, calculate q, w, H, and U for the reaction.arrow_forwardThe octane number of gasoline is based on a comparison of the gasolines behavior with that of 2,2,4-trimethylpentane, C8H18(), which is arbitrarily assigned an octane number of 100. The standard enthalpy of combustion of this compound is 5456.6 kJ/mol. (a) Write the thermochemical equation for the combustion of 2,2,4-trimethylpentane. (b) Use the standard enthalpies of formation in Appendix G to calculate the standard enthalpy of formation of 2,2,4-trimethylpentane.arrow_forwardAcetylene (C2H2) and butane (C4H10) are gaseous fuels with enthalpies of combustion of 49.9 kJ/g and 49.5 kJ/g. respectively. Compare the energy available from the combustion of a given volume of acetylene to the combustion energy from the same volume of butane at the same temperature and pressure.arrow_forward
- A student is asked to calculate the amount of heat involved in changing 10.0 g of liquid bromine at room temperature (22.5C) to vapor at 59.0C.To do this, one must use Tables 8.1 and 8.2 for information on the specific heat, boiling point, and heat of vaporization of bromine. In addition, the following step-wise process must be followed. (a) Calculate H for: Br2(l,22.5C)Br2(l,59.0C) (b) Calculate H for: Br2(l,59.0C)Br2(g,59.0C) (c) Using Hess's law, calculate H for: Br2(l,22.5C)Br2(g,59.0C)arrow_forwardWhat is the heat gained/released at constant pressure equal1o (qp = ?)? What is the heat gained/released at constant volume equal to (qv = ?)? Explain why H is obtained directly from a coffee-cup calorimeter, whereas E is obtained directly from a bomb calorimeter.arrow_forwardCombustion reactions involve reacting a substance with oxygen. When compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. Using the enthalpies of combustion for C4H4 ( 2341 kJ/mol), C4H8 (2755 kJ/mol), and H2 (286 kJ/mol), calculate H for the reaction C4H4(g)+2H2(g)C4H8(g)arrow_forward
- A 220-ft3 sample of gas at standard temperature and pressure is compressed into a cylinder, where it exerts pressure of 2000 psi. Calculate the work (in J) performed when this gas expands isothermally against an opposing pressure of 1.0 atm. (The amount of work that can be done is equivalent to the destructive force of about 1/4 lb of dynamite, giving you an idea of how potentially destructive compressed gas cylinders can be if improperly handled!)arrow_forwardsample of natural gas is analyzed and found to be88.4% methane (CH4) and 11.6% ethane (C2H6) bymass. The standard enthalpy of combustion of methaneto gaseous carbon dioxide (CO2) and liquid water (H2O) is -891 kJ/mol. Write the equation for the combustionof gaseous ethane to carbon dioxide and water.Calculate the standard enthalpy of combustion of ethaneusing standard enthalpies of formation from Table R-11on page 975. Using that result and the standard enthalpyof combustion of methane in Table 15.3, calculate theenergy released by the combustion of 1 kg of natural gas.arrow_forwardStarting with equation 2.27 andthe original definitionof enthalpy, derive the fact that Cp-=Cv-+Rarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
