
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:D
A
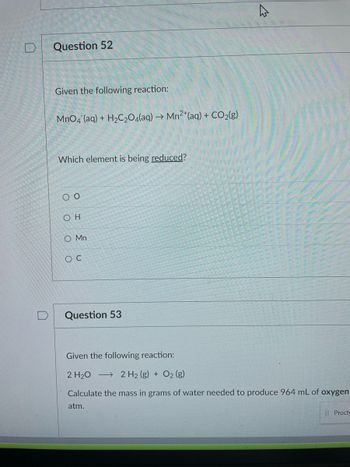
Question 52
Given the following reaction:
MnO4 (aq) + H₂C₂O4(aq) → Mn2+ (aq) + CO₂(g)
Which element is being reduced?
00
ΟΗ
O Mn
OC
Question 53
Given the following reaction:
2 H₂O
2 H2 (g) + O₂ (g)
Calculate the mass in grams of water needed to produce 964 mL of oxygen
atm.
Procte
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to answer all parts. Consider the following balanced redox reaction (do not include state of matter in your answers): 2CrO₂ (aq) + 2H₂O()+6C10 (aq) → 2CrO42 (aq) + 3Cl₂(g) + 40H (aq) (a) Which species is being oxidized? (b) Which species is being reduced? (c) Which species is the oxidizing agent? (d) Which species is the reducing agent? (e) From which species to which does electron transfer occur? Electrons transfer from toarrow_forwardWhich of the following reactions is a combination reaction? Mg (s) + CuSO4 (aq) → MgSO4 (aq) + Cu (s) Na2SO4 (aq) + MgCl2 (aq) → MgSO4 (aq) + 2 NaCl (aq) 2 H2O (g) → 2 H2 (g) + O2 (g) Na2CO3 (aq) + MgCl2 (aq) → MgCO3 (s) + 2 NaCl (aq) 2 H2 (g) + O2 (g) → 2 H2O (g)arrow_forwardWrite the shorthand notation for the following reaction. Mn (aq) + 4 H2O (1) + 5 Ag* (aq) → 5 Ag (6) + MnO4 (aq) + 8 H* (aq)arrow_forward
- Using the reaction 2Al(s) + 3CuCl2·2H2O(aq) -> 3Cu(s) + 2AlCl3(aq) + 6H2O(l), complete the oxidation numbers for each element in the table and determine thearrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 200.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 73.mg . Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO4(aq) → Cu(s) + FeSO4 (aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 111. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. 0-2 X 5arrow_forward
- For each of the following reactions, assign oxidation numbers to each atom/ion and indicate whether the equation represents a redox reaction. If it does, identify the oxidation and reduction components. a) Cu (s) + 2 AgNO3 (aq) → 2 Ag (s) + Cu(NO3)2 (aq) b) 2 NaCl (l) → 2 Na (l) + Cl2 (g) c) 2 C4H10 (g) + 13 O2 (g) → 8 CO2 (g) + 10 H2O (l) d) 2 H2O2 (l) → 2 H2O (l) + O2 (g)arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 300.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 67.mg . Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardWhen the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: HgO (s) →→→ Hg (1) + 0₂ (g)arrow_forward
- Identify the driving force for the chemical reaction: Pb(NO3)2(ag) + 2N2OH (ag) → Pb(OH)2(s) + 2NaNO3 3(aq) O Formation of a precipitate. O Formation of a water neutralization of an acid and base reduction and oxidation. O There is no driving force.arrow_forwardIn the following chemical reaction, which element is the reducing agent? 3 Sn(NO₃)₂(aq) + 2 Fe(s) → 2 Fe(NO₃)₃(aq) + 3 Sn(s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY