
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
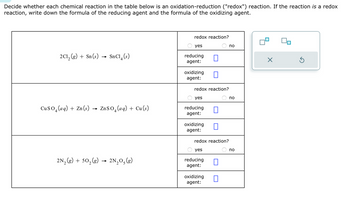
Transcribed Image Text:Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is a redox
reaction, write down the formula of the reducing agent and the formula of the oxidizing agent.
2C1₂(g) + Sn(s) →
SnC14 (s)
CuSO (aq) + Zn (s) → ZnSO₂ (aq) + Cu (s)
4
2N₂(g) + 50₂(g) 2N₂O₂(g)
redox reaction?
yes
reducing
agent:
oxidizing
agent:
redox reaction?
yes
reducing
agent:
oxidizing П
agent:
redox reaction?
yes
reducing 0
agent:
oxidizing 0
agent:
no
no
no
X
00
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The first reaction is of a strong oxidizer (HNO3) with a metal (Cu) to produce copper nitrate, nitrogen dioxide, and water. Indicate the oxidation state of each element (atom) in the reactants and products. Describe which species was oxidized, and which species was reduced in this reaction (where did the electrons come from and where did they go?). (Reaction 1) Cu(s) + HNO3(aq) → Cu(NO3)2(aq) + H2O(l) + NO2(g)arrow_forwardA 4.50 g sample of limestone (impure CaCO3) was dissolved in 0.1M HCl solution according to the following equation. CaCO3(s) + 2HCl(aq) → CaCl2 (aq) + CO2 (g) + H2O (l) Excess of (NH4)2C2O4(aq), was added to the resulting solution to precipitate the calcium ions as calcium oxalate, CaC2O4(s). Ca2+(aq) + C2O42-(aq) → CaC2O4 (s) The precipitate was filtered, dried and weighed at 2.15 g. (Show your work) a) Determine the percentage by mass of calcium carbonate (CaCO3) in the limestone sample. (MM of CaCO3 is 100.09 g/mol) b) Why we need to dry the precipitate until we get a constant mass? c) Is the precipitate of calcium oxalate (CaC2O4) stable and in the final form or not? Explain.arrow_forwardWhen potassium cyanide (KCN) reacts with hydrochloric acid, a poisonous gas of hydrogen cyanide is given off. The equation describing this process is: KCN(aq) + HCL(aq) ==> KCl(aq) + HCN(g) If 0.140 g pf KCN is completely reacted with hydrochloric acid, calculate the amount of HCN that is formed.arrow_forward
- Redox Reactionsarrow_forwardThe following reaction occurs when two aqueous solutions are mixed: Cr(NO3)3(aq) + 3NaOH(aq) → Cr(OH)3(s) + 2NaNO3(aq) Identify the spectator ion OR ions in the solution. SELECT ALL SPECTATOR IONS!arrow_forwardWrite the net ionic equation for the reaction of copper(II) sulfate with sodium sulfide. A) 2 Cu⁺ (aq) + SO₄²⁻ (aq) → Cu₂SO₄ (s) B) 2 Na⁺ (aq) + SO₄²⁻ (aq) → Na₂SO₄ (s) C) Cu²⁺ (aq) + S²⁻ (aq) → CuS (s) D) 2 Cu⁺ (aq) + S²⁻ (aq) → Cu₂S (s)arrow_forward
- When this equation: Fe(s) + AgNO3(aq) → Ag(s) + Fe(NO3)2(aq) is balanced, the net ionic equation is: a. 2Fe(s) + 2Ag*(aq) → 2Ag(s) + 2FE2"(aq) b. 2Fe(s) + Ag*(aq) → Ag(s) + 2FE2"(aq) c. Fe(s) +2 NO; (aq) - → Ae) 2NO3 (i1;) 37. CS Scand F(s, 2Ag"(aq) → 2Ag(s) + Fe“(aq) e. None of the abovearrow_forwardBalance the "molecular" equation for the above reaction: Cu (s) + HNO3 (aq) → Cu(NO3)2 (aq) + NO2 (g) + H20 (1)arrow_forwardConsider the reaction shown below between hydroxide ions (OH), hypochlorite ions (CIO), and chromium hydroxide ions (Cr(OH)4): 2 OH (aq) + 3 CIO (aq) + 2 Cr(OH)4 (aq) → 3 CI (aq) + 5 H₂O (1) + 2 CrO4² (aq) Which atom was oxidized during this reaction? [Select] Which atom was reduced during this reaction? [Select] Which substance was the oxidizing agent in this reaction? [Select] Which substance was the reducing agent in this reaction? [Select]arrow_forward
- W Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. O 2 H* (aq) + CO3²-(aq) → H₂CO3(s) 2 Na* (aq) + CO3²- (aq) + 2 H*(aq) + 2 Cï(aq) → H₂CO3(s) + 2 NaCl(aq) O 2 H* (aq) + CO3²-(aq) → H₂O(1) + CO₂(g) 2 Na* (aq) + CO3²- (aq) + 2 H* (aq) + 2 Cl(aq) → H₂CO3(s) + 2 Na*(aq) + 2 Cl(aq) No reaction occurs.arrow_forwardFor each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. CH4(9)+H₂O(g) → CO(g) + 3 H₂(g) Na₂CO3(s) + H₂PO4(aq) → Na₂HPO4(aq) + CO₂(g) + H₂O(1) 2H,S(aq)+O,(g) →2S(s)+2H,O) FeO(s)+CO(g) → Fe(s)+CO₂(g) Explanation reaction Check highlighted atom is being... oxidized O O O O reduced οιοιοιο O X neither oxidized nor reduced MacBook Air O O S © 2023 McGraw Hill LLC. All Rights Reserved. Terms of U=arrow_forwardBalance the chemical equation below using the smallest possible whole number stoichiometric coefficients. Fe(s) + O₂(g) + H₂O(1) → Fe(OH)₂ (aq) ロ→ロ X 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY