
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Calculate molar mass for delta Tf, molality of t butanol and experimental molar mass of solute. (Kf for t butanol is 8.3°C/m)
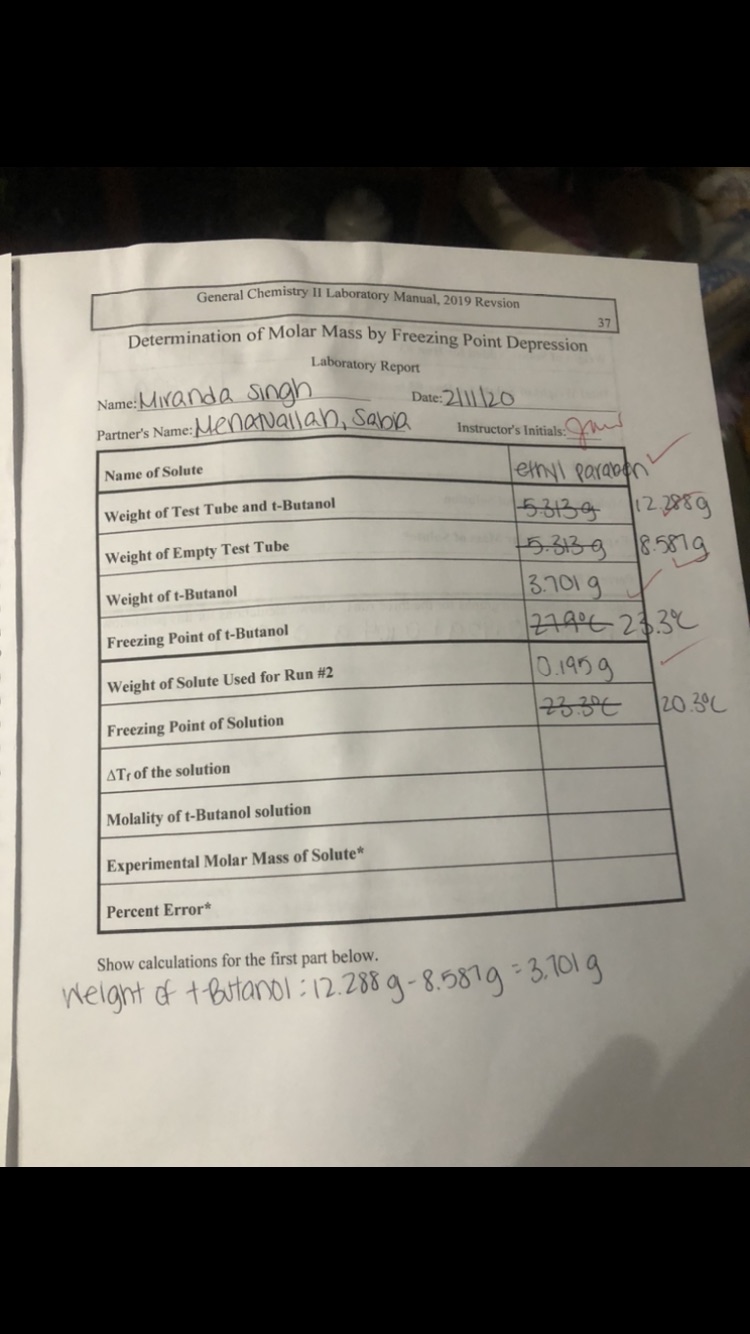
Transcribed Image Text:General Chemistry II Laboratory Manual, 2019 Revsion
Determination of Molar Mass by Freezing Point Depression
37
Laboratory Report
Name:Mivanda Singh
Partner's Name:Menauallan, Sabia
Date: 2||| \20
Instructor's Initials:
Name of Solute
ethyl paraben
12.2859
5-639 18.587g
3.701g
1근구496 243-
0.1959
23.3€
Weight of Test Tube and t-Butanol
Weight of Empty Test Tube
Weight of t-Butanol
Freezing Point of t-Butanol
Weight of Solute Used for Run #2
20.3°C
Freezing Point of Solution
ATr of the solution
Molality of t-Butanol solution
Experimental Molar Mass of Solute*
Percent Error*
Show calculations for the first part below.
Welght of t Butanol :12.288 g-8.5819-3.101 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6-21 Are mixtures of gases true solutions or heterogeneous mixtures? Explain.arrow_forwardA gaseous solute dissolves in water. The solution process has H=15 kJ. Its solubility at 22C and 6.00 atm is 0.0300 M. Would you expect the solubility to be greater or less at (a) 22C and 1 atm? (a) 18C and 6 atm? (a) 15C and 10 atm? (a) 35C and 3 atm?arrow_forwardA solution is made by dissolving 34.0 g of NaCl in 100 g of H2O at 0C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forward
- The Henry's law constant for the solubility of radon in water at is 9.57106 M/mm Hg. Radon is present with other gases in a sample taken from an aquifer at 30C. Radon has a mole fraction of 2.7106 in the gaseous mixture. The gaseous mixture is shaken with water at a total pressure of 28 atm. Calculate the concentration of radon in the water. Express your answers using the following concentration units. (a) molarity (b) ppm (Assume that the water sample has a density of 1.00 g/mL.)arrow_forwardA solution is made by dissolving 0.455 g of PbBr2 in 100 g of H2O at 50C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forward6-111 As noted in Section 6-8C, the amount of external pressure that must be applied to a more concentrated solution to stop the passage of solvent molecules across a semipermeable membrane is known as the osmotic pressure The osmotic pressure obeys a law similar in form to the ideal gas law (discussed in Section 5-4), where Substituting for pressure and solving for osmotic pressures gives the following equation: RT MRT, where M is the concentration or molarity of the solution. (a) Determine the osmotic pressure at 25°C of a 0.0020 M sucrose (C12H22O11) solution. (b) Seawater contains 3.4 g of salts for every liter of solution. Assuming the solute consists entirely of NaCl (and complete dissociation of the NaCI salt), calculate the osmotic pressure of seawater at 25°C. (c) The average osmotic pressure of blood is 7.7 atm at 25°C. What concentration of glucose (C6H12O6) will be isotonic with blood? (d) Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210. mL of solution has an osmotic pressure of 0.953 torr at 25°C. What is the molar mass of lysozyme? (e) The osmotic pressure of an aqueous solution of a certain protein was measured in order to determine the protein's molar mass. The solution contained 3.50 mg of protein dissolved in sufficient water to form 5.00 mL of solution. The osmotic pressure of the solution at 25°C was found to be 1.54 torr. Calculate the molar mass of the protein.arrow_forward
- The solubility of ethylene (C2H4) in water at 20 C and 0.300 atm pressure is 1.27 104 molal. (a) Calculate the Henrys law constant for this gas in units of molal/torr. (b) How many grams of ethylene are dissolved in 1.00 kg water at 20 C if the pressure of the gas is 500 torr?arrow_forwardDistinguish between dispersion methods and condensation methods for preparing colloidal systems.arrow_forwardAn aqueous solution containing 10.0 g of starch per liter has an osmotic pressure of 3.8 mm Hg at 25 C. (a) What is the average molar mass of starch? (The result is an average because not all starch molecules are identical.) (b) What is the freezing point of the solution? Would it be easy to determine the molecular weight of starch by measuring the freezing point depression? (Assume that the molarity and molality are the same for this solution.)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning