
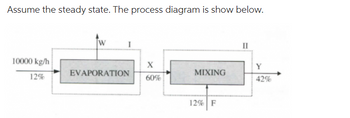
Fresh orange juice with 12% soluble solids content is concentrated to 60% in a multiple effect evaporator (Control surface I). To improve the quality of the final product, concentrated juice (X) is mixed (Control surface I) with an amount of fresh juice so that the concentration of the mixture is 42% (Control surface II).
Considering the overall mass balance and the component mass balance calculate:
a) How much water must be evaporated per hour (W)?
b) How much fresh juice must be added back per hour (F)?
c) How much final product (Y) will be produced per hour, if the inlet feed flow rate is 10,000 kg/h fresh juice?
Assume the steady state. The process diagram is show below.
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 7 images

- Magnesium metal, a gray solid, is heated in a crucible in the presence of oxygen. A white powder is collected from the crucible. This is an example of A) a chemical change B) a separation C) a mixture D) a physical changearrow_forwardThe following consideration(s) is/are relevant to the choice of refrigerant (SELECT ALL that apply): Vapor pressure Environmental factors Toxicity and corrosion properties Water is usually used as the refrigerantarrow_forwardTo make an instant tomato soup, a company makes soup powder from liquid step by a two-step process: concentrating the soup by removing water using a membrane to reach a concentration of 45% water, followed by a spray drying process which leads to a final moisture content of 3%. If the initial liquid tomato soup contains 9.0% solids and fat and enters the membrane separator at 4000 kg/hr, calculate: The flow-rate of the concentrated soup after membrane separation: kg/hr The flow-rate of water removed by the spray drying process (the second stage): kg/hrarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





