
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
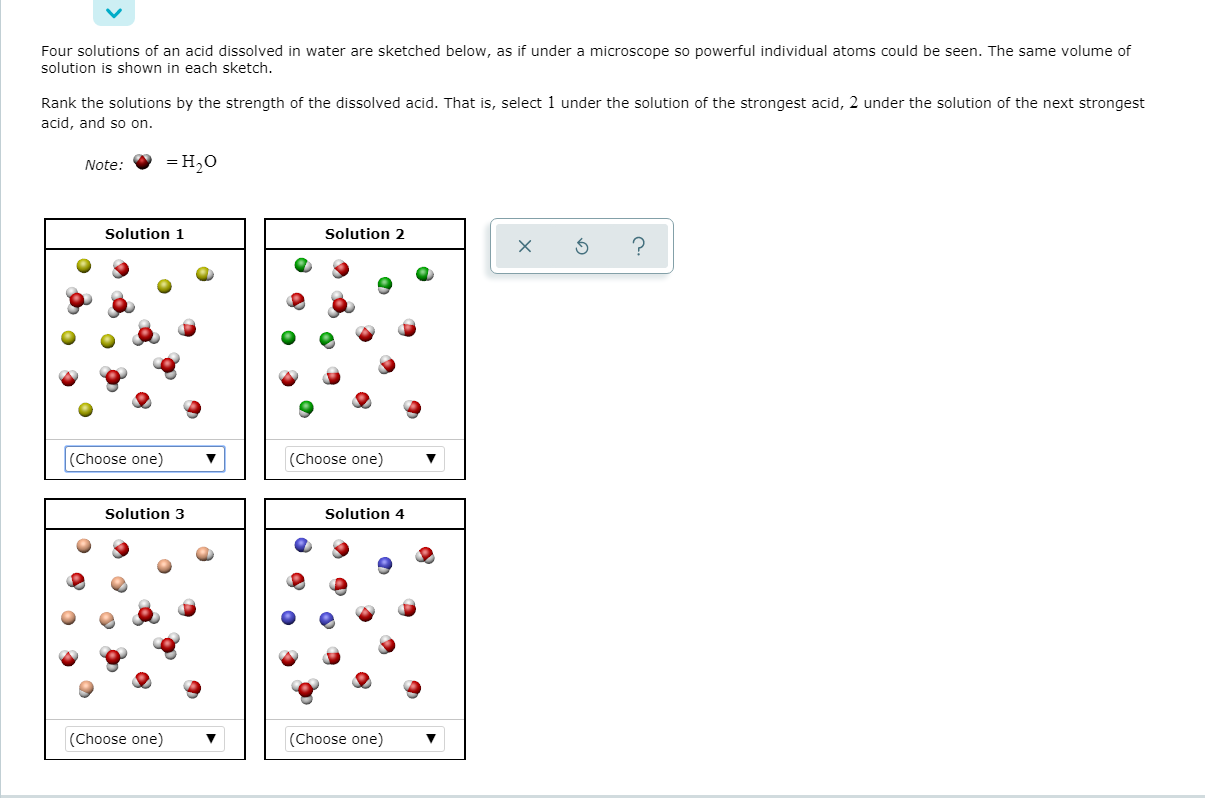
Transcribed Image Text:Four solutions of an acid dissolved in water are sketched below, as if under a microscope so powerful individual atoms could be seen. The same volume of
solution is shown in each sketch.
Rank the solutions by the strength of the dissolved acid. That is, select 1 under the solution of the strongest acid, 2 under the solution of the next strongest
acid, and so on.
Note:
= H,0
Solution 1
Solution 2
(Choose one)
(Choose one)
Solution 3
Solution 4
(Choose one)
(Choose one)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- The chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid HC103 HI H₂PO4 HF strong or weak? strong strong weak weak species present at 10-6 mol/L or greater when dissolved in water П ☐ 0 X 0,0,... Sarrow_forwardCalculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentrations. Indicate which solutions are acidic, basic, or neutral. Complete parts 1-4 below. 1) [OH–] = 8.31×10-3 M pH= pOH= 2) [OH–] = 8.00×10-9 M. pH=. pOH= 3) [H+] = 2.75×10-8 M. pH=. pOH= 4) [H+] = 4.73×10-4 M. pH=. pOH=arrow_forwardEach row of the table below describes an aqueous solution at about 25 °C. Complete the table. That is, fill in any missing entries in the second and third columns. Round your entries for [H3O+] to 2 significant digits, and your entries for pH to 2 decimal places. [H₂O*] solution pH x10 - 4 A 0 ? 8.72 B 0 C mol/L 2.6 × 10 mol/L - 8 7.3 × 10 mol/L X 5arrow_forward
- Please help, i need fast solution. Please don't provide handwritten solution.arrow_forward3:21 2- 3C₂²- + 1 2 3 4 CI Complete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction. NH3(aq) + HCl(aq) (s) Question 1 of 10 O Reset LO 5 6 OH- 7 8 ଇ H H₂O H3O+ ↑ x H₂O Tap here or pull up for additional resources Submit 9 11 (aq) O Narrow_forwardFour solutions of an acid dissolved in water are sketched below, as if under a microscope so powerful individual atoms could be seen. The same volume of solution is shown in each sketch. Rank the solutions by the strength of the dissolved acid. That is, select 1 under the solution of the strongest acid, 2 under the solution of the next strongest acid, and so on. =H₂O Note: Solution 1 (Choose one) Solution 3 (Choose one) Solution 2 (Choose one) Solution 4 (Choose one) X Śarrow_forward
- 1. Part A. H3PO4 is a stronger acid than HClO4 since it has more protons. (True or False) Part B. Water is not an Arrhenius acid but can be a Bronsted acid. (True or False) Part C. A 6 M NaOH (aq) solution has no protons ions (H+) in it. (True or False)arrow_forwardA solution of HCl has a pH of 1.94. How many grams of HCl are there in 298 mL of this solution? g HCI How many grams of HCI are in 298 ml of a HCl solution that has twice the pH? g HCIarrow_forwardplese don't handwritten solution..arrow_forward
- Plz answer each partarrow_forwardcan you help me on the second problem pleasearrow_forwardThe chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid HF H₂PO4 HC1 HBrO3 strong or weak? weak weak strong strong species present at 10-6 mol/L or greater when dissolved in water 0 0 0 X 0,0,... Sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY