
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
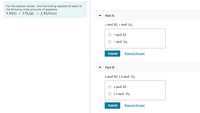
Transcribed Image Text:For the reaction shown, find the limiting reactant for each of
the following initial amounts of reactants.
4 Al(s) + 3 02(9) → 2 Al203(s)
Part A
1 mol Al 1 mo о,
O 1 mol Al
O 1 mol 02
Submit
Request Answer
Part B
4 mol Al, 2.6 mol 02
O 4 mol Al
O 2.6 mol O2
Submit
Request Answer
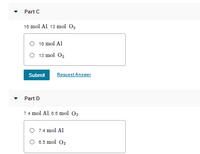
Transcribed Image Text:Part C
18 mol Al 13 mol 02
O 16 mol Al
O 13 mol 02
Submit
Request Answer
Part D
7.4 mol Al 6.5 mol O2
O 7.4 mol Al
O 6.5 mol O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the following reaction, how many grams of potassium sulfate are required for the complete reaction of 21.2 grams of barium chloride? barium chloride (aq) + potassium sulfate(aq) → barium sulfate(s) + potassium chloride (aq) Mass= garrow_forwardFor the following reaction, 37.3 grams of sulfuric acid are allowed to react with 42.5 grams of zinc hydroxide. sulfuric acid (aq) + zinc hydroxide(s) → zinc sulfate (aq) + water (1) What is the maximum amount of zinc sulfate that can be formed? Mass= What is the formula for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Mass= g.arrow_forward[Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forward
- How much (in gram) of HCl is required to produce 21.45 g of AlCl3 if the percentage yield for the following reaction is 61.24% ? Enter your answer without units. Molar masses (in g/mol):Al = 26.98H2 = 2.016HCl = 36.46AlCl3 = 133.34 2Al(s) + 6 HCl (aq) ⟶⟶ 2 AlCl3(s) + 3H2(g)arrow_forwardFor the following reaction, 17.5 grams of iron are allowed to react with 37.0 grams of chlorine gas. iron(s) + chlorine(g) → iron(III) chloride(s) What is the maximum mass of iron(III) chloride that can be formed? Mass = 9 What is the FORMULA for the limiting reactant? What mass of the excess reagent remains after the reaction is complete? Mass= garrow_forwardFor the following reaction, 58.6 grams of potassium hydroxide are allowed to react with 37.9 grams of phosphoric acid potassium hydroxide ( aq ) + phosphoric acid ( aq ) potassium phosphate ( aq ) + water ( I ) What is the maximum amount of potassium phosphate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams Submit Answer Try Another Version 3 item altempts remainingarrow_forward
- For the following reaction, 16.7 grams of chlorine gas are allowed to react with 8.90 grams of water.3 Cl2(g) + 3 H2O(l) 5 HCl(aq) + HClO3(aq) What is the FORMULA for the limiting reagent? What is the maximum mass of hydrochloric acid that can be formed? gramsWhat mass of the excess reagent remains after the reaction is complete? gramsarrow_forwardBalance each reaction, then suppose exactly 13.3 g of each reactant is taken. Indicate which reactant is the limiting reagent. Calculate the mass of each product that is expected. Al(s) + Cl2(g) → AlCl3(s)limiting reagent AlCl2 AlCl3 producedarrow_forwardi dont get how to do any of thisarrow_forward
- Aluminum chloride, AlCl3, is made by treating scrap aluminum with chlorine.2 Al(s) + 3 Cl2(g) → 2 AlCl3(s)If you begin with 7.80 g Al and 3.11 g Cl2, identify the limiting reactant and determine what mass of the excess reactant remains when the reaction is completed?arrow_forwardThe simple reaction A(s) + 2B(s) → 3C(s) has a theoretical yield of 17.2 g C. What is the percent yield when 12.72 g of C are isolated?arrow_forwardIn an experiment, 12.4 g of KClO3 were carefully decomposed, and 4.52 of O2 gas were collected. What are the theoretical, actual, and percent yields of the reaction? The unbalanced chemical reaction is given below. KClO3 → KCl + 3 O2 Was the percent yield greater or less than 100%? What are at least three reasons for the percent yield not being exactly 100%?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY