
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For the reaction in which calcium metal reacts with water to form Ca(OH)2 and H2 (g), solve the following: How many water molecules will be needed to react with 10.1 g of calcium?
Expert Solution
arrow_forward
Step 1

arrow_forward
Step 2
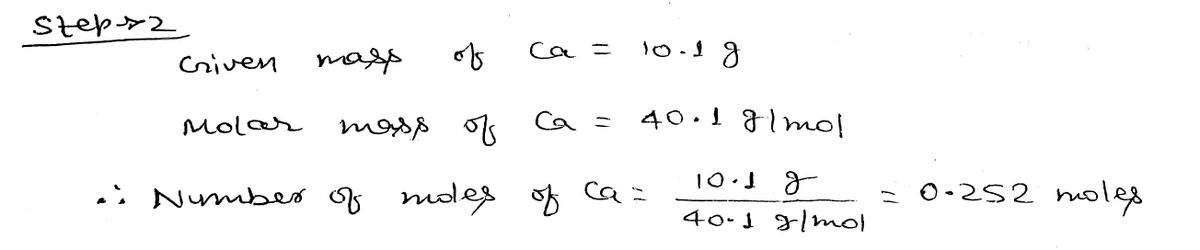
arrow_forward
Step 3
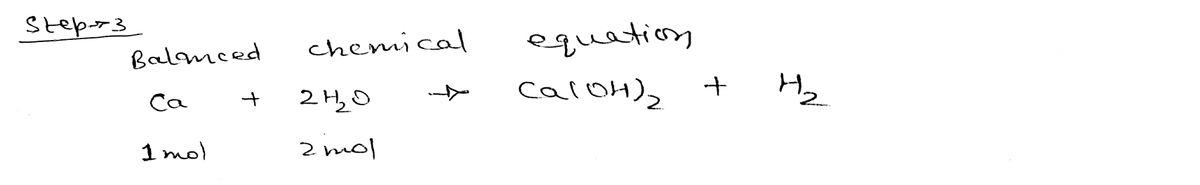
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predicting the reactants of a neutralization reaction Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → KC1O₂(aq) + H₂O(1) X 0-0 Ś ? olo 18 Ar Earrow_forwardThe balanced equation below shows a simple way of manufacturing hydrogen gas in lab (you've done thisl). For your convenience, the molar mass of each substance is shown below their formulas (in purple). Use this information to make the requested calculation: 2 Al(s) + 6 HCI(aq) -> 2 AICI3(aq) + 3 H2(g) 26.982 36.461 133.341 2.016 What volume of hydrogen gas, in mL, would be produced by the reaction of 922 mg of hydrochloric acid with excess aluminum metal?arrow_forwardSolid copper can be produced by passing gaseous ammonia over solid copper (II) oxide at high temperatures, according to the following reaction. NH3 (g) + CuO (s) → N2 (g) + Cu (s) + H2O (g) Balance the reaction.arrow_forward
- Aqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: “Never mix ammonia and bleach, as toxic gases may be produced.” One of the toxic gases that can be produced is chloroamine, NH2Cl.Another toxic gas that can be produced is nitrogen trichloride, NCl3. What is the oxidation number of N in nitrogen trichloride?arrow_forwardIf 200 kJ of heat are supplied, how many grams of NO can be produced? N2(g) + O2(g)→ 2 NO(g) AH = 180.6 kJ/mol O 66.5 g 54.2 g O 30.1 g O 2.21 garrow_forwardAn aqueous solution of NaF and an aqueous solution of PbCl2 react. Predict what solid will form and balance the chemical equation. If 30.6 mL of NaF solution reacted completely with excess PbCl2 to produces 10.7 g of the precipitate. What is the molarity of the original NaF solution?arrow_forward
- For the following reaction, 0.557 moles of potassium hydroxide are mixed with 0.224 moles of phosphoric acid. potassium hydroxide(aq)+phosphoric acid(aq)= potassium phosphate(aq)+water(l) What is the formula for the limiting reagent? What is the maximum amount of potassium phosphate that can be produced?arrow_forward6. Sodium sulfide reacts with hydrochloric acid to produce hydrogen sulfide and sodium chloride. How many grams of sodium sulfide are required for complete reaction with 15.0 mL of 0.250 M hydrochloric acid? The molar mass of sodium sulfide is 78.046 g/mol. (Hint: start by writing the balanced equation for the reaction.)arrow_forward1. Label each of the following reactions as combination (synthesis), decomposition, single replacement, or double replacement. Then complete and balance the following reactions. Reaction Type of reaction? Redox or nonredox? H2(g) + 02 (g) → K (s) + N2 (g) → C (s) + 02 (g) → H2CO3 (aq) → electrical current H₂O (1) 300°C Au2O3 (s) 300°C Ag2O (s) → NH3 (g) → Pb (s) + Cu(NO3)2 (aq) → Na (s) + HOH (1) -> BaBr2 (aq) + Na3PO4 (aq) → H2SO4 (aq) + NH4OH(aq) → Cl2 (g) + NaI (aq) → Na2S (s) + HCl(aq) → Fe (s) + 02 (g) →arrow_forward
- Because of the toxicity of mercury compounds, mercury(I) chloride is used in antibacterial salves. The mercury(I) ion Hg22+ consists of two bound Hg+ ions.How many grams of mercury(I) chloride are needed to saturate 4900 km3 of water (the volume of Lake Michigan)? (Ksp = 1.5 × 10−18) .arrow_forwardUse the chemical equation and table to answer the following question. Which mass of water is expected to be produced in the reaction? Question 4 options: 18.0 g because the mass of the products must equal the mass of the reactants. 76.5 g because the mass of the products must equal the mass of the reactants. 18.0 g because mass is created to form the products. 76.5 g because mass cannot be created or destroyed.arrow_forwardA sample of 8.51 g of solid calcium hydroxide is added to 35.0 mL of 0.480 M aqueous hydrochloric acid. Write the balanced chemical equation for the reaction. Physical states are optional. chemical equation: What is the limiting reactant? hydrochloric acid calcium hydroxide How many grams of salt are formed after the reaction is complete? mass of salt: How many grams of the excess reactant remain after the reaction is complete? excess reactant remaining: garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY