
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
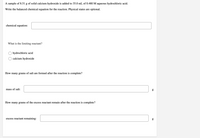
Transcribed Image Text:A sample of 8.51 g of solid calcium hydroxide is added to 35.0 mL of 0.480 M aqueous hydrochloric acid.
Write the balanced chemical equation for the reaction. Physical states are optional.
chemical equation:
What is the limiting reactant?
hydrochloric acid
calcium hydroxide
How many grams of salt are formed after the reaction is complete?
mass of salt:
How many grams of the excess reactant remain after the reaction is complete?
excess reactant remaining:
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- For the following reaction, 0.370 grams of hydrogen gas are allowed to react with 52.0 grams of iodine. hydrogen (g) + iodine (s) →→→→→→ hydrogen iodide (g) What is the maximum amount of hydrogen iodide that can be formed? What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams gramsarrow_forwardChapter 4 Limiting reactantsarrow_forwardFor the following reaction, 3.77 grams of chlorine gas are mixed with excess iron. The reaction yields 4.82 grams of iron(III) chloride. iron (s) + chlorine (g) →→→→→→→iron(III) chloride (s) grams What is the theoretical yield of iron(III) chloride What is the percent yield for this reaction ? %arrow_forward
- . Consider a 3.52-g sample of CaCO3 (99.87% pure) in a flask and a 100.0 mL sample of vinegar (5% acidity) in a graduated cylinder. The combined mass of both reagents and containers is 255.98 g. After swirling the reaction mixture for about twenty minutes, the combined mass of the reaction mixture and containers is found to be 254.46 g. What is the percent yield of carbon dioxide in this experiment?arrow_forwardMagnesium metal reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen gas. When 7.30 g of magnesium metal is added to 100.0 mL of 3.00 M hydrochloric acid solution, what mass of hydrogen gas is produced, assuming the reaction goes to completion? a) Not enough information is provided. b) 0.302 g c) 0.605g d) 0.247 g e) 0.151 garrow_forward1.Does a reaction occur when aqueous solutions of chromium(III) chloride and sodium hydroxide are combined? yes or no If a reaction does occur, write the net ionic equation. 2. write a balanced equation for the combination reaction described, using the smallest possible integer coefficients.When carbon (graphite) combines with oxygen , carbon dioxide is formed.arrow_forward
- of 15 An aqueous solution containing 9.88 g of lead(II) nitrate is added to an aqueous solution containing 5.48 g of potassium chloride. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. balanced chemical equation: What is the limiting reactant? O lead(II) nitrate O potassium chloride The reaction goes to completion, but in the process of washing and drying the precipitate, some was lost. The percent yield for the reaction is 84.8%. How many grams of precipitate are recovered? F precipitate recovered: R V G Search or type URL % 5 T G B MacBook Pro 6 Y H & 7 N U J 8 00 M 1 ( 9 K O V H I ) O L P ^. { لا لا / 1 = ? 11 1 miarrow_forward1) Group 1 metals react almost instantly and violently with water, as a single replacement reaction to produce an aqueous solution of metal hydroxide and hydrogen gas. A sample of solid lithium weighing 84.25 mg is dropped into a beaker containing 50.0 mL of water. Assume the density of water is 0.9988 g/mL. a) Write the balanced equation. b) Calculate the theoretical yield (in grams) of lithium hydroxide. c) Assuming the reaction is complete, and that there is no volume change, what is the concentration (in M) of lithium hydroxide in the solution that results?arrow_forwardAqueous solutions barium chloride and potassium sulfate are combined. 1) Predict the products and write the balanced equation showing the states of each substance. 2) The barium chloride solution has a volume of 100.0 mL and contains 10.00 grams of barium chloride. The molarity of the potassium sulfate solution is 0.574 moles/liter and has a volume of 100.0 mL. Determine the limiting reactant and the mass of each product in grams. 3) Determine how many grams of excess reactant remain. 4) Calculate the molarity of just the potassium ions after the reaction has finished.arrow_forward
- for the following reaction 28.3 grams of disphosphorus pentoxide are allowed to react with 15.0 grams of water. What is the maximum amount of phosphoric acid that can be formed?arrow_forwardConsider the following reaction: Solid calcium oxide reacts with carbon dioxide gas to form solid calcium carbonate. A chemist allows 14.4g of calcium oxide and 13.8g of carbon dioxide to react. When the reaction is finished, the chemist collects 19.4g of calcium carbonate. Determine the limiting reagent, theoretical yield, and percent yield.arrow_forwardFor the following reaction 36.4 grams of sulfuric acid are allowed to react with 40.1 grams of zinc hydroxide. What is the maximum amount of zinc sulfate that can be formed? What is the formula for the limiting reagent? What amount of the excess reagent remains after the reaction is complete?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY