
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:19
Preq
2req
2req
s 2req
s 2req
Ots 2req
#
3
E
D
THIC NO Calculations
C
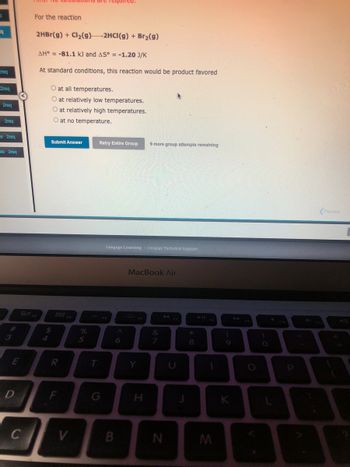
For the reaction
2HBr(g) + Cl₂(g) 2HCI(g) + Br₂(g)
AH = -81.1 kJ and AS° = -1.20 J/K
At standard conditions, this reaction would be product favored
80,
O at all temperatures.
O at relatively low temperatures.
O at relatively high temperatures.
O at no temperature.
Submit Answer
$
R
F
V
%
5
T
Retry Entire Group 9 more group attempts remaining
Cengage Learning Cengage Technical Support
6
B
MacBook Air
Y
H
&
7
N
*
8
1
M
(
K
O
F10
P
E
E
Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. Which of the following technologies are considered non-renewable energy sources? Choose ALL that apply. a. Coal Power Plants b. C. Hydrogen Fuel Cells Fossil Fuel Power Plants a. b. C. 8. Which of the following reactions are endothermic? Choose ALL that apply. H2(g) + 1/2O2(g) H₂O(g); AH = -243 kJ/mol ¹1/2N2(g) + ¹//202(g) + 90.7 kJ → NO(g) CO(g) + 111 kJ C(s) + ¹1/202(g) d. Hydroelectric Generators Solar Panels e. f. Wind Turbines a. Acid lonization Constant (K₂) b. Base lonization Constant (K) C. Equilibrium Constant (Keg) d. e. f. HI(g)→→ ¹1/₂2H2(g) + ½l2(g); AH = −26 kJ/mol H₂O(g) · H2(g) + ½O2(g); AH = +243 kJ/mol + O2(g) 2NO N2(g) + 181.4 kJ (g) 9. Which equilibrium constant would be most relevant to the following system in equilibrium: HC₂H3O2(aq) → H*( (aq) + C₂H3O₂ (aq) Partial Pressure Equilibrium Constant (K₁) d. e. Solubility Product Constant (Ksp) f. Water lon Product Constant (Kw)arrow_forwardThe reaction 4AI (s) + 302 (g)→ 2 Al203 (s) AH° = -3351 kJ is and therefore heat is by the reaction. thermoneutral, neither released nor absorbed O endothermic, absorbed exothermic, released exothermic, absorbed endothermic, releasedarrow_forwardoptions for all: 1. Reactants. 2. Products.arrow_forward
- Methane-producing bacteria convert liquid acetic acid (CH3CO₂H) into CO₂(g) and CH₂(g). AS° ΔΗ, Ο (kJ/mol) (J/mol.K) CH3CO₂H (1) -484.5 159.8 CO₂(g) -393.5 213.8 CH₂(g) -74.8 186.2 Calculate AHOrxn = kJ Calculate AG rxp = (round to 3 sig figs) Is this process endothermic or exothermic under standard conditions? Is the reaction spontaneous under standard conditions? kJ (round to 3 sig figs)arrow_forward16. Use enthalpies of formation given to determine the standard enthalpy of reaction for the following: ed oioege Al2O3 (s) +2 Fe 2 Al (s) + Fe2O3 (s) AH° =??? > AH°r(kJ/mol) -825.5 -1676 17. Use the enthalpies of formation and the enthalpy of reaction given below to determine the enthalpy of formation for solid CaC2. CaC2 (s) + 2 H2O (1) → Ca(OH)2 (s) + C2H2 (g) AH° =-127 kJ AH° (kJ/mol) ??? -286 -986 +227arrow_forwardplease help?arrow_forward
- Consider the reaction. vopica! Submit Answe Use the References to access important values if needed for this question. 2SO₂(g) + O₂(g) 2503(g) Using the standard thermodynamic data in the tables linked above, calculate AGxn for this reaction at 298.15K if the pressure of each gas is 11.74 mm Hg. ANSWER: kj/mol Retry Entire Group 2 more group attempts remainingarrow_forwardA east.cengagenow.com 山 OWLV2 | Online teaching and learning resource from Cengage Le... [Review Topics] [References] Use the References to access important values if needed for this questlon. For the reaction H2(g) + C,H,(g) C,H,(g) AG° =-103.0 kJ and AS° = -120.7 J/K at 282 K and 1 atm. The maximum amount of work that could be done by this reaction when 2.35 moles of H,(g) react at standard conditions at this temperature is kJ. Submit Answer Retry Entire Group 1 more group attempt remaining In progress Next jiarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Methane-producing bacteria convert liquid acetic acid (CH³CO₂H) into CO₂(g) and CH₂(g). AS° ΔΗ, Ο (kJ/mol) (J/mol.K) CH3CO₂H (1) -484.5 159.8 CO₂(g) -393.5 213.8 CH₂(g) -74.8 186.2 Calculate AHºrxn = kJ Calculate AG, = rxn (round to 3 sig figs) Is this process endothermic or exothermic under standard conditions? Is the reaction spontaneous under standard conditions? kJ (round to 3 sig figs)arrow_forwardHeat of reaction (AH°) for the following reaction at 25.0 °C is Fe;O4(6) + CO(g) 3FEO(s) + CO2(g) Given the following Heat of Formation: AHF0304(s) = (-1118 kJ/mol), AHCO(g) = (-110.5 kJ/mol), AHF«06) = (-272KJ/mol), AHco2(g) ( -393.5 kJ/mol),arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY