
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For the preparation of methyl iodide, 200 lbs/day of Hydroiodic acid are added to excess methanol.
HI + CH3OH → CH3I + H2O
If the product contains 81.6 % along with the unreacted methanol and waste contains 82.65% HI and 17.35 % H20, calculate, assuming that the reaction is 40% complete in the vessel;
Find the values of:
a. X
b. R
c. Y
d. W
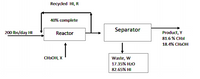
Transcribed Image Text:Recycled HI, R
40% complete
Separator
200 Ibs/day HI
Reactor
Product, Y
81.6 % CH3I
18.4% CНОН
CH3OH, X
Waste, W
17.35% H20
82.65% HI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- can you help me solve this, this is what i have so far but is there a different way of doing this. please type solution in word. calculate how much KNO3 can be removed as solid (filtered) from a 1-kilogram solution of 60 wt% KNO3 in water (40 wt%) at 65 °C, when it is cooled to 25 °C. Note that the 60 wt% value is given on a basis of total solution mass, which is different than the values presented in the graph.arrow_forwardA barytes composed of 100 percent BaSO4 is fused with carbon in the form of cokecontaining 6 percent ash (which is infusible). The composition of the fusion mass is:BaSO4 11.1%BaS 72.8%C 13.9%Ash 2.2 100% Reaction: BaSO4 + 4 C → BaS + 4 COFind the :4.1 Excess reactant. (10)4.2. The percentage of the excess reactant. (3)4.3. The degree of completion of the reaction. ?arrow_forwardFastarrow_forward
- Allyl chloride is used as a precursor in the production of allyl alcohol, glycerin, and a variety of other products used in the pharmaceutical industry. Suppose you have designed a reactor to produce allyl chloride via the thermal chlorination of propylene: C3H6 + Cl2 -> C3H5Cl + HCl In an undesired side reaction, propylene can react with chlorine to produce dichloropropene. C3H6 + 2Cl2 -> C3H4Cl2 + 2HCl The reactor is designed to yield a 25% conversion of propylene and a selectivity of 13 mol C3H5Cl/mol C3H4Cl2. For the reactive system described above, calculate the molar compositions of the reactor feed and product streamsarrow_forwardAniline is produced by the hydrogenation of nitrobenzene. A small amount of cyclohexylamine is produced as a by-product. Nitrobenzene is fed to the reactor as a vapor with three times the required stoichiometric amount of hydrogen. The conversion of nitrobenzene to the products is 96% and the selectivity to aniline is 85%. Unreacted hydrogen is separated from the reaction products and recycled to the reactor. From the recycle line it is purged to keep the inerts in the recycle stream below 5%. The fresh hydrogen that is fed is 99.5% pure and the rest is inert. Calculate the adiabatic outlet temperature of the reaction products and indicate the relationship with respect to the reference temperature (298.15) (in K):arrow_forwardW.W. Heckert et E. Mack studied the decomposition of ethylene oxide in gas phase at 25°C (C₂H4O → CH4 + CO), and found that it is a first-order reaction in the reactant. Determine the reaction rate constant using the date given below, for a reaction that begins with a pure reactant: time (min) Ptot (torr) 0 115.30 6 122.91 7 124.51 8 126.18 10 129.10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The