
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
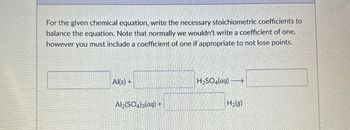
Transcribed Image Text:For the given chemical equation, write the necessary stoichiometric coefficients to
balance the equation. Note that normally we wouldn't write a coefficient of one,
however you must include a coefficient of one if appropriate to not lose points.
Al(s) +
H2SO4(aq) →
Al2(SO4)3(aq) +
H2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Predict the possible products for each reaction. Use the solubility rules to predict the phase of each product. Write the phase for each product. Balance the complete chemical equation. Write the formula equation, complete ionic equation and the net ionic equation ____Fe(NO3) 3 (aq) + ____Na2S (aq) ->arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. 0 - H,0(1) NaF (aq) +arrow_forwardwrite the Formula Equation for the reaction between solutions of silver perchlorate and calcium bromide using the observed results for two other reactions shown below. Your answer must contain the correct subscripts and superscripts. AgClO4(aq) + NaBr(aq) -> yields precipitate CaBr2(aq) + KClO4(aq) -> no reactionarrow_forward
- The balanced equation below shows a simple way of manufacturing hydrogen gas in lab (you've done this!). For your convenience, the molar mass of each substance is shown below their formulas (in purple). Use this information to make the requested calculation: 2 Al(s) + 6 HCI(aq) -> 2 AICI3(aq) + 3 H2(g) 26.982 36.461 133.341 2.016 How much hydrochloric acid, in g, would we need to make 5.00 L of hydrogen gas in lab using this reaction?arrow_forwardBalance and write the net ionic equation for the following reaction Mg(s) + AgNO3(aq) → Mg(NO3)2(aq) + 2Ag(s) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:arrow_forwarda Choose the correct balanced equation that describes this precipitation reaction: Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + NaCl(aq) Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + 2NaCl(ag) Naz CO3 (aq) + CaCl2 (ag) → CaCO3 (aq) + 2NACI(ag) Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + NaCl(ag) Naz CO3 (ag) + CaCl2 (ag) → CaCO3 (ag) + NaCl(ag) b Choose the correct balanced equation that describes this precipitation reaction: Fe(C,H3O2)2 (aq) + Na, S(ag) → FeS(s) + NaC, H3 O2 (aq) Fe(C2H3 O2)2(ag) + Na2S(aq) → FeS(aq) + NaC, H3 O2 (aq) Fe(C, H3 O2)2 (ag) + Naz S(ag) → FeS(s) + NaC, H3 O2 (aq) Fe(C, H3O2)2 (aq) + Naz S(ag) → FeS(aq) + 2N2C, H3 02 (aq) Fe(C,H3O2)2 (ag) + Na2S(aq) → FeS(s) + 2NAC, H3O2 (ag) c Choose the correct balanced equation that describes this precipitation reaction: KOH(ag) + FeCl2(aq) → Fe(OH)2(s) + KCI(aq) 2КОН(ад) + FeCl, (aq) Fe(ОH)2(ag) + 2KCI(ag) 2КОН (ад) + FeCl, (aq) > Fe(ОH)2 (8) + 2КCI(aд) 2КОН (аq) + FeClz (aq) -> Fe(ОH)2 (8) + КKСІ(ад) о конад) + FeCl, (ag) - Fe(ОH)2(8) +…arrow_forward
- Choose the correct complete ionic equation and total ionic equation for the following reaction. Lithium carbonate reacts with magnesium chloride. O Mg2+ (aq) + 3CO² (aq) → MGCO3 (s) O Mg2+ (aq) + C (aq) + 302 (aq) → MgCO, (s) O Liz* (aq) + CO32 (aq) + Mg2+ (aq) + Cl2 (aq) → MgC03 (s) + Liz* (aq) + Cl, (aq) O2LI* (aq)+ C032 (aq) + Mg²* (aq) + 2Cl (aq) MgCO3 (s) +2Li* (aq) + 2CI (aq) O Mg2* (aq) + Co, (aq) MgCO3 (s) O Li* (aq) + CO32- (aq) + Mg2* (aq) + CI (aq) → MGCO3 (s) +Li* (aq) + CI (aq)arrow_forward1. Write a balanced complete ionic equation for the reaction and a balanced net ionic equation for the reaction. H3PO4(aq)+HNO3(aq)→ 2. Write a balanced complete ionic equation for the reaction. Al(NO3)3(aq)+NaOH(aq)→ 3.Write a balanced complete ionic equation for the reaction. HI(aq)+Pb(NO3)2(aq)→arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. → CaC1,₂(aq) + H₂O(1) X Śarrow_forward
- a student is given 50.0 mL of a solution of Na2CO3 of unknown concentration. to determine the concentration of the solution, the student mixes the solution with excess 1.0 M CA(NO3)2 (aq), causing a precipitate to form. the balanced equation for the reaction is: Na2CO3 (aq) + Ca(NO3)2 -> 2 NaNO3 (aq) + Ca CO3 (s) a) write the net ionic equation for the reaction that occurs when the solutions of Na2CO3 and Ca (NO3)2 are mixed. B) the diagram attached is incomplete. Draw in the species needed to accurately represent the major ionic species remaining in the solution after has been completed.arrow_forwardWrite the net ionic equation for the reaction when solutions of magnesium sulfide (MgS) and copper (1) nitrate (CUNO3) are mixed given the reaction below: Reaction: MgS (aq) + 2 CuNO3 (aq) → Mg(NO3)2(aq) + Cu₂S (s) Be sure to write out the complete ionic reaction first to find which ions are spectator ions.arrow_forwardThe following molecular equation represents the reaction that occurs when aqueous solutions of silver(I) nitrate and magnesium chloride are combined.2AgNO3 (aq) + MgCl2 (aq) 2AgCl (s) + Mg(NO3)2 (aq)Write the balanced net ionic equation for the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY