
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
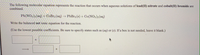
Transcribed Image Text:The following molecular equation represents the reaction that occurs when aqueous solutions of lead(II) nitrate and cobalt(II) bromide are
combined.
Pb(NO3)2(aq) + CoBr2 (aq) → PbBr2 (s) + Co(NO3)2 (aq)
Write the balanced net ionic equation for the reaction.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- write the net ionic equation for the reaction in the photoarrow_forwardThe concentration of SO42– ions in a 45.0 mL sample of seawater is determined by adding a solution of BaCl2 and precipitating the SO42– as BaSO4. After the precipitate is filtered from the solution, it is dried and weighed. If the mass of BaSO4 recovered is 0.315 g, what is the sulfate concentration of the seawater sample? Express your answer in mmol/L.arrow_forwardI don't get it. I need help with this questionarrow_forward
- Apply the concepts of stoichiometry and solution concentration in this section. Potassium iodide reacts with lead (II) nitrate in the following precipitation reaction 2 KI (aq) + Pb(NO3)2 (aq) KNO3 (aq) + PbI2 (s). What volume of 0.200 M KI solution is required to completely precipitate all of the lead in 255mL of a 0.312 M Pb(NO3)2 solution? An solution is made by dissolving 28.4 g of glucose (C6H12O6) in 355 g of water. The final volume of the solution is 378 mL. Calculate the molarity, molality, mole fraction, mole percent and mass percent of the solution, Hydrochloric acid is usually purchased in a concentrated form that is 37.0 % HCl by mass and has a density of 1.20 g/mL. Describe exactly how to prepare 2.85 L of a 0.500 M HCl to be used in Dr. Henary’s CHEM1211K lab from the concentrated solution. Hint (Find molarity of concentrated solution and use dilution equation. This is how your solution are prepared)arrow_forwardConsider the following unbalanced precipitation reaction: CuBr2 (aq) + KOH (aq) → KBr(aq) + Cu(OH)2 (s) Provide the net ionic equation for this reaction. I will be looking for charges, subscripts, coefficients, and states. The X2 button can be used to provide superscripts and X2 button can be used to provide subscripts.arrow_forwardThe traditional method of analysis for the amount of chloride ion present in a sample is to dissolve the sample in water and then slowly to add a solution of silver nitrate. Silver chloride is very insoluble in water, and by adding silver nitrate, it is possible effectively to remove all chloride ion from the sample. Ag+(aq) + Cl-(aq) → AgCl(s) Suppose a 1.515 g sample is known to contain 27.5% chloride ion by mass. What mass of silver nitrate must be used to completely precipitate the chloride ion from the sample?arrow_forward
- Fe(II) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts Fe(II) to insoluble Fe(III): $$4Fe(OH)+(aq)+4OH−(aq)+O2(g)+2H2O(l)4Fe(OH)3(s) How many grams of O2 are consumed to precipitate all of the iron in 75.0 mL of 0.0350 M Fe(II)?arrow_forwardComplete and balance the equation for the single displacement reaction between sodium and lead (II) nitrate. Phases are optional. Balanced equation: Na(s)+Pb(NO3)2(aq) =arrow_forwardHow many millilitres of 0.450 mol L−1 H2SO4(aq) are required to completely neutralize 46.7 mL of 0.930 mol L−1 KOH(aq) ? Hint: Write a balanced chemical equation for the reaction. Give your answer in mL, accurate to three significant figures. Do not include units as part of your answer.arrow_forward
- For each of the following reactions, suggest two soluble ionic compounds that, when mixed together in water, result in the net ionic equation given: (a) 2 Ag+ (aq) + CO3²¯ (aq) → Ag₂CO3(s) (b) Mg²+ (aq) + 2 OH¯(aq) → Mg(OH)₂(s), the suspension present in milk of magnesia 3+ (c) 3 Ca³+ (aq) + 2 PO2 (aq) → Ca3(PO4)2(s), gypsum, a component of concretearrow_forwardFe(II) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts Fe(II) to insoluble Fe(III): 4Fe(OH)* (aq) +40H (aq) + O₂ (g) + 2H₂O(1)→→→→→→ 4Fe(OH)3 (s) How many grams of O₂ are consumed to precipitate all of the iron in 70.0 mL of 0.0550 M Fe(II)?arrow_forwardWrite the net ionic equation for the reaction when solutions of magnesium sulfide (MgS) and copper (1) nitrate (CUNO3) are mixed given the reaction below: Reaction: MgS (aq) + 2 CuNO3 (aq) → Mg(NO3)2(aq) + Cu₂S (s) Be sure to write out the complete ionic reaction first to find which ions are spectator ions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY