
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
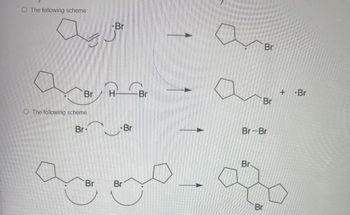
Transcribed Image Text:The following scheme:
-Br
Br
Br
H
Br
The following scheme
Br
Br
Br
Br
+
-Br
Br
Br-Br
Br
Br
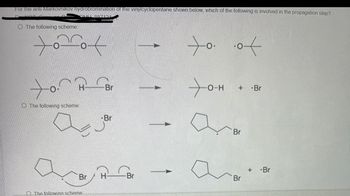
Transcribed Image Text:For the anti-Markovnikov hydrobromination of the vinylcyclopentane shown below, which of the following is involved in the propagation step?
4
NOT
O The following scheme:
SMUL 997121
to of
to of
to
H
Br
+
-O-H + -Br
The following scheme:
The following scheme
Br
а
Br
Br
H
Br
Br
+ -Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Plastic photochromic sunglasses are based on the following reversible rearrangement of a dye inside the lenses that occurs when the lenses are exposed to sunlight. The original dye absorbs UV light but not visible light and is thus colorless, while the rearrangement product absorbs visible light and is thus darkened. (a) Show the mechanism of the rearrangement. (b) Why does the rearrangement product absorb at a longer wavelength (visible light) than the original dye (UV)?arrow_forwardBenzvl chloride can be converted into benzaldehvde by treatment with nitromethane and base. The reaction involves initial conversion of nitromethane into its anion, followed by SN2 reaction of the anion with benzyl chloride and subsequent Ε2 reaction. Write the mechanism in detail, using curved arrows to indicate the electron flow in each step.arrow_forwardNeed help on this questionarrow_forward
- RA Provide the missing major product, and the mechanism (SN1, SN2, E1, E2) for each case below. Show stereochemistry and regiochemistry where appropriate.d bios odi lsded 9onimslydiom Mechanism CH MH enimslyrtiem HO bios oionsqoig e.A = 6 2. 'Br (babon dinm on) muindiliups 16 benovsi sd bluow zbuborg NaOCH3 in refluxing methanol OTs ČH,CH3 nood ldsia tasol ori bns oldste om ordi lodedarrow_forwardmost stable to least stable nucleophilesarrow_forwardDetermine the mechanism of nucleophilic substitution of each reaction and draw the productincluding stereochemistry.arrow_forward
- For the reaction below, draw he reachon mechanisms) (aurows) and predict al she major products. If no reachon will H2C acur brey explain whag: Br CH20Harrow_forwardGive the major substitution product of the following reaction. Br O CH3CO₂H Save for Later heat OH OMe ? There is no reaction under these conditions or the correct product is not listearrow_forwardPlease don't provide handwriiten solution...arrow_forward
- In EAS bromination reactions, a -NHCOCH3 substituent on the aromatic ring is: O an activator and an o,p-director. O a deactivator and a m-director. an activator and a m-director. NHCOCH3 catalyzes the 1,2 addition of bromine across the double bond O a deactivator and an o,p-director.arrow_forwardIdentify the expected major product of the following electrocyclic reaction. → OI Oll O III OIV OV A ? KYYAK 8 +En ||| + En I || IV + En Varrow_forwardConsider the following mechanism of reaction: CH3 CH, CH3 CH;C CH, + H-CI CH;CCH, + C: → CH;CCH, tert-butyl cation CI tert-butyl chloride In this reaction: The alkene and the carbocation intermediate are both nucleophilic HCl and CI" are both nucleophilic The alkene and HCI are both electrophilic The carbocation and CI" are both electrophilic The alkene and CI" are both nucleophilic AMeving to another question will save this responsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
