
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
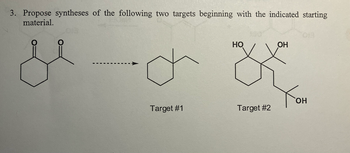
Transcribed Image Text:3. Propose syntheses of the following two targets beginning with the indicated starting
material.
013
HO
OH
Тон
Target #1
Target #2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Indicate the main product(s) expected to be obtained in the following reactions.arrow_forwardWhat would be the major product(s) of the following reaction? །། Brtw HO 20°C (CH3)2CHCH20 I (CH3)2CHCH20 O A. I, II, and III OB. IV SOC. III OD. None of these choices. OE. I and II II III (CH2CHCH20 ཡ་ཀ IVarrow_forwardContent 54°F Clear * X app.101edu.co Draw the major product of the substitution reaction shown below. Ignore any inorganic byproducts. F1 Aktiv Chemistry. @ 2 CH3CO2H Draw SN1 Product F2 # 3 DII X OSN2/SN1 Reactions Fl: X G Which alkyl bromide i X F3 $ ▬ F4 % 5 J F5 A 6 Question 8 of 22 â 6 F6 & G whats a stro 7 Please select a c F7 O PrtScn 8arrow_forward
- 3. Fill in the boxes to propose syntheses for the following molecules. OHarrow_forward3) Consider the synthesis below. Identify P1 and deduce What are the reagents/reactants A and B A NH₂ A = B P1 B ii) Zn/(Hg) HC1 HN Brarrow_forwardChoose reagents to convert 2-cyclohexenone to the following compounds. Syntheses may require several steps. Use letters from the table to list reagents in the order used (first at the left). i 1. Li(CH2=CH)2Cu Reagents a 1. Li(CH3)2Cu 2. H3O+ e 1. Li(C6H5)2Cu 2. H3O+ b 1. NaBH4 f CH2l2/Zn(Cu) / ether j 2. H3O+ C NH3 / KOH g 1. CH3MgBr/dry ether k 2 H3O+ d H2NNH2/KOH h HN(CH3)2 HO CH3 a) b) OH N(CH3)2 2. H3O+ (C6H5)3P+-CH2 H₂ over Pd/C KMnO4/H3O+arrow_forward
- Chemistry CH3 o-nitrotoluene CH3 NH CI NO2 NH,HCO, 10% Pd/C Step 1 1) NaOAc, H₂O ayla CI CH3 o-toluidine NH3*,CI CH3 CI 4 2-chloro-N-o-tolilpropanami 2) Step 2 2 o-toluidine assignment I need complete mechanism IZarrow_forward#5arrow_forwardSelect the appropriate reagent from the reagent bank to accomplish each step in the reactions below. Reaction 1: Reaction 2: Reaction 3: Reaction 4: G Reaction 1 НО. Reaction 2 lo Reaction 3 iOH OH Reaction 4 CN NH₂ ? ? . ? ? НО. loh OH lot NH₂ HO 1) BH3THF 2) H3O+ A HNEt₂ E 1) Et₂CuLi 2) H₂O I mCPBA M Reagent Bank ta NaH B HCI H₂O F HNEt₂ Trace H+ J SOCI₂ N CH3OH H* C 1) EtMgBr 2) H₂O G H₂C=PPh3 K H₂NEt Trace H* O 1) LIAIH4 2) H₂O D 38 НО. H* H -OH H* L DCC OH Parrow_forward
- Alcohols are important for organic synthesis, especially in situations involving alkenes. The alcohol might be the desired product, or the OH group might be transformed into another functional group via halogenation, oxidation, or perhaps conversion to a sulfonic ester derivative. Formation of an alcohol from an alkene is particularly powerful because conditions can be chosen to produce either the Markovnikov or non-Markovnikov product from an unsymmetrical alkene. Using your reaction roadmap as a guide, show how to convert 4-methyl-1-pentene into 5-methylhexanenitrile. You must use 4-methyl-1-pentene and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forwardChoose reagents to convert 2-cyclohexenone to the following compounds.arrow_forwardIn both examples below the reactants shown are combined to bring about a nucleophilic substitution (SN1, Sn2) and/or elimination (E1, E2) reaction. What is the major reaction that takes place in each case? CH3 NaOCH2CH3 CH,CH,CCH3 CH;CH,OH SN2 E2 mixture of SN1 and E1 NaSCH3 CH3OH CH2Iarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning