
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
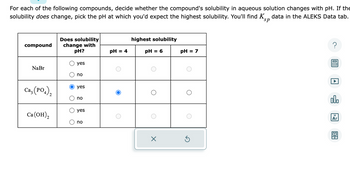
Transcribed Image Text:For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the
solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab.
sp
Does solubility
highest solubility
compound
change with
pH?
pH = 4
pH = 6
pH = 7
NaBr
yes
no
Ca, (PO4)2
Ca(OH)2
yes
no
yes
no
☑
?
000
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- Sketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forwardYou have a solution of the weak acid HA and add some of the salt NaA to it. What are the major species in the solution? What do you need to know to calculate the pH of the solution, and how would you use this information? How does the pH of the solution of just the HA compare with that of the final mixture? Explain.arrow_forwardSketch a pH curve for the titration of a weak acid (HA) with a strong base (NaOH). List the major species, and explain how you would go about calculating the pH of the solution at various points, including the halfway point and the equivalence point.arrow_forward
- Consider the nanoscale-level representations for Question 111 of the titration of the aqueous strong acid HA with aqueous NaOH, the titrant. Water molecules and Na+ ions are omitted for clarity. Which diagram corresponds to the situation: (a) After a very small volume of titrant has been added to the initial HA solution? (b) Halfway to the equivalence point? (c) When enough titrant has been added to take the solution just past the equivalence point? (d) At the equivalence point? Nanoscale representations for Question 111.arrow_forwardThe weak base ethanolamine. HOCH2CH2NH2, can be titrated with HCl. HOCH2CH2NH2(aq)+H3O+(aq)HOCH2CH2NH3+(aq)+H2O(l) Assume you have 25.0 mL of a 0.010 M solution of ethanolamine and titrate it with 0.0095 M HCl. (Kb for ethanolamine is 3.2 107.) (a) What is the pH of the ethanolamine solution before the titration begins? (b) What is the pH at the equivalence point? (c) What is the pH at the halfway point of the titration? (d) Which indicator in Figure 17.11 would be the best choice to detect the equivalence point? (e) Calculate the pH of the solution after adding 5.00, 10.0, 20.0, and 30.0 mL of the acid. (f) Combine the information in parts (a), (b), (c), and (e), and plot an approximate titration curve.arrow_forwardAcrylic acid is used in the polymer industry in the production of acrylates. Its K, is 5.6 X 10“’. What is the pH of a 0.11 M solution of acrylic acid, CH2CHCOOH?arrow_forward
- A buffer is prepared in which the ratio [ H2PO4 ]/[ HPO42 ]is 3.0. (a) What is the pH of this buffer? (b) Enough strong acid is added to convert 15% of HPO42- to H2PO4-. What is the pH of the resulting solution? (c) Enough strong base is added to make the pH 7.00. What is the ratio of [H2PO4-] to [HPO42-] at this point?arrow_forwardWhen a diprotic acid, H2A, is titrated with NaOH, the protons on the diprotic acid are generally removed one at a time, resulting in a pH curve that has the following generic shape: a. Notice that the plot has essentially two titration curves. If the first equivalence point occurs at 100.0 mL NaOH added, what volume of NaOH added corresponds to the second equivalence point? b. For the following volumes of NaOH added, list the major species present after the OH reacts completely. i. 0 mL NaOH added ii. between 0 and 100.0 mL NaOH added iii. 100.0 mL NaOH added iv. between 100.0 and 200.0 mL NaOH added v. 200.0 mL NaOH added vi. after 200.0 mL NaOH added c. If the pH at 50.0 mL NaOH added is 4.0, and the pH at 150.0 mL NaOH added is 8.0, determine the values Ka1, and Ka2 for the diprotic acid.arrow_forwardYou have a solution of the weak acid HA and add some HCl to it. What are the major species in the solution? What do you need to know to calculate the pH of the solution, and how would you use this information? How does the pH of the solution of just the HA compare with that of the final mixture? Explain.arrow_forward
- When a diprotic acid. H2A. is titrated with NaOH, the protons on the diprotic acid are generally removed one at a time, resulting in a pH curve that has the following generic shape: a. Notice that the plot has essentially two titration curves. If the first equivalence point occurs at 100.0 mL NaOH added, what volume of NaOH added corresponds to the second equivalence point? b. For the following volumes of NaOH added, list the major species present after the OH reacts completely. i. 0 mL NaOH added ii. between 0 and 100.0 mL NaOH added iii. 100.0 mL NaOH added iv. between 100.0 and 200.0 mL NaOH added v. 200.0 mL NaOH added vi. after 200.0 mL NaOH added c. If die pH at 50.0 mL NaOH added is 4.0 and the pH at 150.0 mL NaOH added is 8.0, determine the values Ka and Ka. for the diprotic acid. d.arrow_forwardThe simplest amino acid is glycine, H2NCH2CO2H. The common feature of amino acids is that they contain the functional groups: an amine group, -NH2, and a carboxylic acid group, -CO2H. An amino acid can function as either an acid or a base. For glycine, the acid strength of the carboxyl group is about the same as that of acetic acid. CH3CO2H, and the base strength of the amino group is slightly greater than that of ammonia, NH3. (a) Write the Lewis structures of the ions that form when glycine is dissolved in 1 M HCl and in 1 M KOH. (b) Write the Lewis structure of glycine when this amino acid is dissolved in water. (Hint: Consider the relative base strengths of the -NH2 and -CO2- groups.)arrow_forwardYou are given the following acidbase titration data, where each point on the graph represents the pH after adding a given volume of titrant (the substance being added during the titration). a What substance is being titrated, a strong acid, strong base, weak acid, or weak base? b What is the pH at the equivalence point of the tiration? c What indicator might you use to perform this titration? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning