
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question

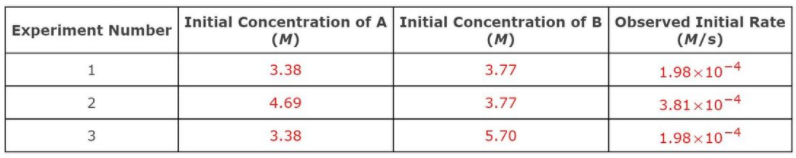
Transcribed Image Text:For a reaction A + B → products, the following data were collected.
Initial Concentration of A Initial Concentration of BObserved Initial Rate
(M)
Experiment Number
(M)
(M/s)
1
3.38
3.77
1.98x10-4
2
4.69
3.77
3.81x10-4
3
3.38
5.70
1.98x10-4
Calculate the rate constant for this reaction.
4.0
|m-1.s-1
Expert Solution
arrow_forward
Step 1
The reaction taking place is given as,
=> A + B ------> Products
And the reaction kinetics data given is,
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11.102 Suppose that you are studying a reaction and need to determine its rate law. Explain what you would need to measure in order to accomplish this in a single experiment, and how you could use graphical methods to get from the experimental data to a complete rate law.arrow_forwardA friend of yours states, A balanced equation tells us how chemicals interact. Therefore, we can determine the rate law directly from the balanced equations. What do you tell your friend?arrow_forwardCandle wax is a mixture of hydrocarbons. In the reaction of oxygen with candle w ax in Figure 11.2, the rate of consumption of oxygen decreased with time after the flask was covered, and eventually' the flame went out. From the perspective of the kinetic-molecular theory, describe what is happening in the flask. FIGURE 11.2 When a candle burns in a closed container, the flame will diminish and eventually go out. As the amount of oxygen present decreases, the rate of combustion will also decrease. Eventually, the rate of combustion is no longer sufficient to sustain the flame even though there is still some oxygen present in the vessel.arrow_forward
- The rate law for a reaction can be determined only from experiment and not from the balanced equation. Two experimental procedures were outlined in Chapter 12. What are these two procedures? Explain how each method is used to determine rate laws.arrow_forwardConsider the following hypothetical data collected in two studies of the reaction 2A+2BC+2D Time(s) Experiment 1 [A] (mol/L) Experiment 2 [A] (mol/L) 0 1.0 102 1.0 102 10. 8.4 103 5.0 103 20. 7.1 103 2.5 103 30. ? 1.3 103 40. 5.0 103 6.3 104 In Experiment 1, [B]0 = 10.0 M. In Experiment 2, [B]0 = 20.0 M. Rate=[A]t a. Use the concentration versus time data to determine the rate law for the reaction. b. Solve for the value of the rate constant (k) for the reaction. Include units. c. Calculate the concentration of A in Experiment 1 at t =30.sarrow_forwardThe rate law for a reaction can be determined only from experiment and not from the balanced equation. Two experimental procedures were outlined in Chapter 11. What are these two procedures? Explain how each method is used to determine rate laws.arrow_forward
- Experimental data are listed here for the reaction B: Time (s) IB] (mol/L) 0.00 0.000 10.0 0.326 20.0 0.572 30.0 0.750 40.0 0.890 Prepare a graph from these data, connect the points with a smooth line, and calculate the rate of change of [B] for each 10-s interval from 0.0 to 40.0 s. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result. How is the rate of change of [AJ related to the rate of change of [B] in each time interval? Calculate the rate of change of [AJ for the time interval from 10.0 to 20.0 s. What is the instantaneous rate, A[B]/Ar, when [BI = 0.750 mol/L?arrow_forwardThe reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) was studied at 904 C, and the data in the table were collected. (a) Determine the order of the reaction for each reactant. (b) Write the rate equation for the reaction. (c) Calculate the rate constant for the reaction. (d) Find the rate of appearance of N2 at the instant when [NO] = 0.350 mol/L and [H] = 0.205 mol/L.arrow_forwardThe initial rate for a reaction is equal to the slope of the tangent line at t 0 in a plot of [A] versus time. From calculus, initial rate = d[A]dt . Therefore. the differential rate law for a reaction is Rate = d[A]dt=k[A]n. Assuming you have some calculus in your background, derive the zero-, first-, and second-order integrated rate laws using the differential rate law.arrow_forward
- Consider a hypothetical reaction between A and B: A + B products Use the following initial rate data to calculate the rate constant for this reaction. [A] (mol/L) [B] (mol/L) Initial Rate (mol/L s) 0.20 1.0 3.0 0.50 1.0 11.8 2.0 2.0 189.5arrow_forwardTwo mechanisms are proposed for the reaction 2NO(g)+O2(g)2NO2(g)Mechanism 1: NO+O2NO3(fast) NO3+NO2NO2(slow) Mechanism 2: NO+ON2O2(fast) N2O2+O22NO2(slow) Show that each of these mechanisms is consistent with the observed rate law: rate=k[ NO2 ]2[ O2 ].arrow_forwardA study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a) Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s. (b) Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate? (c) Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning 
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning