
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can you help me get the missing products. Drawn out in extended form please?
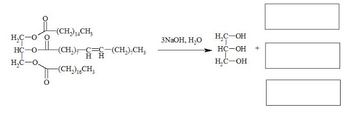
Transcribed Image Text:H₂C-O O
IⅡ
-0
H₂C-O.
-(CH₂) 14CH3
(CH₂) C-C-(CH₂),CH,
H H
-(CH₂) 16CH3
3NaOH, H₂O
H,C-OH
HC-OH
H₂C-OH
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- he Claisen condensation converts two molecules of an ester into a ß-keto ester. The reaction starts with the ester in an koxide/alcohol solution and is worked up with acid to form the neutral ß-keto ester product. ih 1. "OCH/CH₂/CH/CH,OH 2.HO how the curved-arrow mechanism for the Claisen condensation of ethyl ethanoate treated with ethoxide ion. Include all formal harges and nonbonding electrons. In each step, draw only the species that react in that step. If an enolate resonance form is ossible, draw only the carhanionic form. If a CH, group is being deprotonated, draw all H's on the reacting site. Tip: always mit ethanol byproducts. Step 1. Add curved arrows. / Draw CH Draw Templates Draw • Templates 11 II Step 2. Draw the ester-containing intermediate produced from step 1, and draw the next reactant or reagent, il applicable. Add curved arrows and any necessary charges and nonbonding electrons. Templat More H More 11 Step 3. Draw the ester-containing intermediate produced from step…arrow_forwardPlease help me with final productarrow_forward1. Label each of the following carboxylic acid derivatives with the functional group name. Then, fill in the necessary reagent(s) over/next to each arrow in the following scheme. R CI R R R OH R OR' R NR'2arrow_forward
- Using chemdraw or a any chemical drawing program show the mechanism of the decarboxylation of 3,4- Dihydroxyhydrocinnamic acid (DHHCA). Be sure to show the m/z (mass to charge ratios) of the starting material and decarboxylated product.arrow_forwardHello can someone tell me if this will work for this reagent please helparrow_forwardCan the product shown below be made by a Michael addition? If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area. +2 1. Na Me 2.H" work up وید You can draw 1 and 2 in any arrangement you like. Click and drag to start drawing a structure. Can't be done with a Michael addition. × G ☐: P 무arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. CH3NH2, TSOH Drawing Problem 66 of 18 Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Remove Copy Drag To Pan Reset Submit Done Paste +1arrow_forward1. Draw the structure of each substance. has been done for you to get you started. Then answer the following questions about all the substances you will be using in this lab practical. Limonene has been done for Draw in the boxes below the structures that correspond to the different kercises substances. you to get you started. that we will be investigating. (R)-(+)-Limonene (S)-(-)-Limonene CH3 CH3 TLC H2CH CH2 hemeiry C am H2C used cr H2C CH3 CH3 CLimonene Limonene (skeletal form) Eugenol Carvone platearrow_forwardshow full and complete procedure. Do not skip any step. please HANDWRITTEN ONLYarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardplease help with the correct steps to get this productarrow_forwardDraw the product, please ก D L. CH,CH,CH,Mg Br 2. H₂O 1. H₂O* 2. PCC J. CH,MgBr 4. HO LHCN KEN 2. H,O Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY