
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
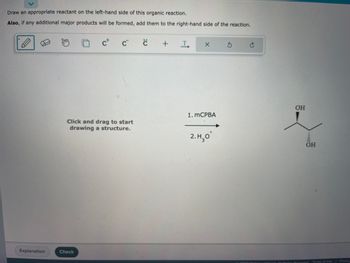
Transcribed Image Text:Certainly! Below is a transcription suitable for an educational website:
---
**Organic Reaction Assignment**
**Instructions:**
Draw an appropriate reactant on the left-hand side of this organic reaction.
**Note:** If any additional major products will be formed, add them to the right-hand side of the reaction.
**Reaction Details:**
The reaction involves two steps:
1. mCPBA (meta-Chloroperoxybenzoic acid)
2. \( \text{H}_3\text{O}^+ \) (Hydronium ion)
**Expected Product:**
The product of the reaction depicted is a molecule with two hydroxyl groups (OH) on adjacent carbon atoms, indicating the formation of a diol. This configuration is typical in the conversion of alkenes to 1,2-diols through an epoxidation and hydrolysis sequence.
**Diagram Instructions:**
On the left side, click and drag to start drawing a structure that would react under the given conditions to lead to the 1,2-diol product shown.
**Tools Available:**
- A pencil for drawing bonds and structures.
- Options to add charges, radicals, and text annotations.
- Undo and redo buttons for correcting mistakes.
**Functionality:**
- After drawing the proposed reactant, click “Check” to validate your structure.
- Use the "Explanation" button for detailed feedback.
---
This transcription provides users with clear guidance on how to engage with the exercise, details about the chemical reaction process, and the purpose of the interactive tools provided in the image.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Make two identical models of methane with four different colored balls attached to each carbon. On one of the models switch two of the balls. Draw the two structures in stereoscopic projection (with dash, wedge, etc.). Label each hydrogen as H1, H2, etc.arrow_forwardhelp with identifying the functional groups A-Harrow_forwardPlease don't provide handwritten solutionarrow_forward
- 11:51 l 5G Question 9 of 25 Submit 1. O3 2. CH3SCH3 Select to Draw CSH100 Select to Draw CH2Oarrow_forwardPart II (70 points) Directions: Answer each of the questions that follow. Please read the directions for each question carefully. When structures are required please draw them Clearly and Neatly. Make sure questions are numbered clearly as well. 1. (12 points; 2 points each) Give a correct name for each of the following compounds: CH3 A) CH—CH,C—CHICHCHCH, CH OH alf B) CH-CH₂- CH₂ -CH₂-CH₂arrow_forwarddetermine the IHD and circle the functional groupsarrow_forward
- Consider the mechanism of the acid-catalyzed cleavage of the ether shown below. excess HBr heat Br Br O Complete the mechanism by adding curved arrows and products. Add steps as necessary and be sure to add lone pairs and charges where relevant. 田 C to :0 + Add/Remove step Xarrow_forwardWhich of the following illustrates an equatorial OH? HO OH OH OA O A. 현 B. C. D.arrow_forward. Circle and label all of the non-alkane functional groups. Be as specific as possible (use 1o, 2o and 3o to describe amines and amides). Then, draw the line angle structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY