
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Five molecules are presented in Figure 1 above. How many of the molecules contain one or more
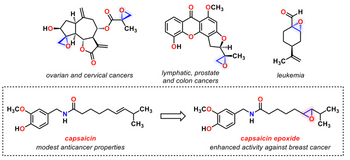
Transcribed Image Text:H3CO.
CH3
ကိုယ်ပုံ
OH
HO
HO
ovarian and cervical cancers
CH3
OCH3
CH3
lymphatic, prostate
and colon cancers
H3CO
gogling goghing
`N
H
H
capsaicin
capsaicin epoxide
enhanced activity against breast cancer
modest anticancer properties
H
HO
CH3
H3C
H
leukemia
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Are my answers right? Can you help me more understand how to classify if its primary, secondary, and tertiaryarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward7. Circle each of the following examples below that have acceptable curved arrows. For those that are circled, draw the products made by the given curved arrows. For those that are not, explain why in 1-3 complete sentences. Do not add any additional curved arrows. HO (Br H-OH₂ Br BrAlBr3 Фон -NH3 - Larrow_forward
- answer number 3 onlyyarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward3. Complete the following scheme by showing the key hydrogen atoms (protons) and using curved arrows to clearly show electron movements in all mechanistic steps. B OHarrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 MgBr Will the first product that forms in this reaction create a new CC bond? 1。 MgBr 00 Yes ○ No ? Will the first product that forms in this reaction create a new CC bond? ○ Yes ○ No. Click and drag to start drawing a structure. ✗ ח' :arrow_forwardPlease answer in detail with explanation and step by steparrow_forwardHello, can you help me with this question? Please make sure your answer is not wrong. Thank you.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY