
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
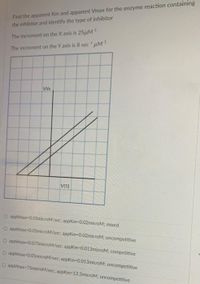
Transcribed Image Text:Find the apparent Km and apparent Vmax for the enzyme reaction containing
the inhibitor and identify the type of inhibitor
The increment on the X axis is 25uM
The increment on the Y axis is 8 sec uM
We
VISI
O appvmax-0.05microM/sec; appKm=0.02microM: mixed
O appvmax-0.05microM/sec; appKm=0.02microM; uncompetitive
O appvmax-0.075microM/sec, appKm-0.013microM; competitive
O appvmax-0.05microM/sec; appKm-0.013microM; uncompetitive
O appvmax 75microM/sec; appKm-13.3microM; uncompetitive
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. Graphing the results from kinetics experiments with enzyme inhibitors The following kinetic data were obtained for an enzyme in the absence of inhibitor (1), and in the presence of two different inhibitors (2) and (3) at 5 mM concentration. Assume [Er] is the same in each experiment. [S] (mM) 1 2 4 8 12 1 2 4 8 12 [S] (mm) a. Determine Vmax and Km for the enzyme. b. Determine the type of inhibition and the Kıfor each inhibitor. 1/[S] M-1 Plot these data as double-reciprocal Lineweaver-Burk plots and use your graph to answers a. and b. 1000 500 250 125 83.3 Answer: The data may be analyzed using double-reciprocal variables. For each [S] and corresponding u, we will calculate 1/[S] and 1/v. sed to get mo Helmien (1) v (μmol/mL sec 12 20 29 35 40 1/v (1) v (1) μmol/mL se mL-sec/umol C 12 20 29 35 40 Please explain how to get K 1/v 20 × 104 10 × 104 -400 8.33x104 5.00x104 3.45x104 2.86x104 2.50x104 Condition No inhibitor 0 5 mM inhibitor (2) 5 mM inhibitor (3) Plots of 1/v vs. 1/[S]…arrow_forwardAn atomic absorption method for the determination of the amount of iron present in used jet engine oil was found from pooling 30 triplicate analyses to have a standard deviation s = 3.1 µg Fe/mL. If s is a good estimate of o, calculate the 95 and 99% confidence intervals for the result 21.7 µg Fe/mL if it was based on the following criteria. (a) a single analysis 95% confidence interval= 21.7 + 99% confidence interval = 21.7 + (b) the mean of two analyses 95% confidence interval = 21.7 + 99% confidence interval= (c) the mean of six analyses 21.7 ± 95% confidence interval = 21.7 + 99% confidence interval= 21.7 + μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mL μg Fe/mLarrow_forwardThe students conducted the assay for LDH activity using the two serum samples. Each cuvette (path length 1 cm) contained 3ml of a suitable assay buffer (including pyruvate as substrate). 20 microlitres of either serum was added to the cuvette and the absorbance values immediately recorded at the optimum wavelength for a period of 5 minutes (absorbance readings taken every 30 seconds). Protein concentration of serum sample (mg/ml) Change in absorbance at optimum wavelength per minute Control serum (C) 8 -0.04 Diseased serum (D) 7.8 -0.6 1c. Using the molar absorption coefficient of NADH as 6220 M-1 cm-1, and by application of the Beer-Lambert law, estimate the enzyme activity in the two samples (C and D). Express activity as moles per second. 1d. Estimate the specific activity of the two samples (moles per second per microgram).arrow_forward
- From the following data (ESSAY DPPH & METHANOL), make a graph of the adjusted model Concentration (x-axis) vs. % inhibition (y-axis), provide the equation that is given and explain its behavior in a broad way. Do this activity step by step pleasearrow_forwardWhen the reactions in part (B) are repeated in the presence of 12 ?M of an uncompetitive inhibitor, the y - intercept of the Lineweaver - Burk plot is 0.352 (?mol? 1mls). Calculate K? for this inhibitor. I tried 0.187 and it was off. Not sure what units to use either.arrow_forwardIf inhibitor X changes lactase activity to a Vo of 0.10 mM per minute when [S] = 1.0 mM, and a Vo of 0.133333333333 mM per minute when [S] = 2.0 mM, calculate the new Vmax. Calculate the new Km of the above lactase reaction, with inhibitor X. Calculate the slope on a Lineweaver-Burk plot (Km / Vmax) for the lactase reaction with inhibitor X. Inhibitor X exerts which of the following effects on the above enzyme (lactase)?arrow_forward
- Only typed solutionarrow_forwardCalculate the slope on a Lineweaver-Burk plot (Km / Vmax) for the lactase reaction with inhibitor X. (inhibitor X changes lactase activity to a Vo of 0.10 mM per minute when [S] = 1.0 mM, and a Vo of 0.133333333333 mM per minute when [S] = 2.0 mM) 0.20 per minute 0.50 per minute 1.0 per minute 2.0 per minute 5.0 per minutearrow_forwardIR PAP Chemnistr x G diagram show X PAP Chemistry x G What is the be x E PAP Chemistr x E PAP Chemistr x -a Session Timex Mp Rockin a ar-2903012.agilixbuzz.com/student/135113422/activity/c412d902-b23e-4202-b813-76a1f9f4c196 Mastery Assess It 8 que Solis PAP Chemistry-2903012-42100P-1/Le Chatelier's Principle / Lesson 128 All changes saved 8. A chemist tested the reaction of potassium chlorate (KCIO3) and Iron (II) sulfate (FeSo). The Independent varlable is temperature; all other variables are constant. The results were recorded. Temperature (degrees Kelvin) Reaction Rate (grams per second) 285 290 295 These results support which conclusion? O An increase in temperature increassed the mass of each molecule of reaction product. An increase in temperature increased the amount of reactant present. O An increase in temperature increased the catalytic behavior of reactants. O An increase in temperature increased the rate of collision of reactant molecules. PREVIOUS 8 of 25 NEXT SAVE & EXIT…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY