
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:IR PAP Chemnistr x G diagram show X
PAP Chemistry x
G What is the be x
E PAP Chemistr x
E PAP Chemistr x
-a Session Timex
Mp Rockin
a ar-2903012.agilixbuzz.com/student/135113422/activity/c412d902-b23e-4202-b813-76a1f9f4c196
Mastery Assess It 8
que
Solis
PAP Chemistry-2903012-42100P-1/Le Chatelier's Principle / Lesson 128
All changes saved
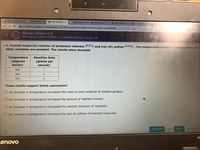
8. A chemist tested the reaction of potassium chlorate (KCIO3) and Iron (II) sulfate (FeSo). The Independent varlable is temperature; all
other variables are constant. The results were recorded.
Temperature
(degrees
Kelvin)
Reaction Rate
(grams per
second)
285
290
295
These results support which conclusion?
O An increase in temperature increassed the mass of each molecule of reaction product.
An increase in temperature increased the amount of reactant present.
O An increase in temperature increased the catalytic behavior of reactants.
O An increase in temperature increased the rate of collision of reactant molecules.
PREVIOUS
8 of 25
NEXT
SAVE & EXIT
N:
enovo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me answer these subunits!arrow_forwardWhy does the rate of most reactions decrease over time? A. A decrease in reactant concentration reduces the frequency of successful collisions. B. A decrease in temperature reduces the average kinetic energy of the particles. C. An increase in product concentration interferes with the effectiveness of the catalyst. D. An increase in pressure of the system reduces the movement and energy of particles.arrow_forwardHydrogen peroxide decomposes as shown below: 2 H2O2 → 2 H2O + O2 molar mass H2O2 = (34.0 g/mol) A sample of hydrogen peroxide solution was analyzed using the procedure from the “Enzymatic Decomposition and Analysis of Hydrogen Peroxide” experiment. A 7.54 g sample of the solution generated 0.0277 mol of oxygen gas. Calculate the mass percent of H2O2 in the solution.arrow_forward
- Please send me the question in 20 minutes it's very urgent plzarrow_forward12.A decomposition reaction can be represented by the following reaction:Immersive reader A)CaCO3 -------------> CaO + CO2 B) Cu + H2SO4--------->CuSO4 + H2 C) HCl + NH3----------->NH4Cl D) S + O2---------------->S02 E) None of the above.arrow_forwardIron fillings (very small pieces of iron) rust faster than iron nails. This demonstrates that the rate of reaction depends on the a. temperature of the reactants b. chemical nature of the reacting substances c. state of subdivision of the reactants d. concentrations of the reactants e. presence of a catalystarrow_forward
- The following represents the effect of time on the concentration during a reaction. The upper line represents a reactant concentration and the lower line the concentration of a product. Which of the following statements is not correct? Concentration B C Time → O Between points A and D the reactant concentration is decreasing. O Between points B and C the product concentration is increasing. At point D the rate of the forward and reverse reactions are the same. O At point C the rate of product breakdown equals that of reactant consumption.arrow_forwardUsing the collision model for a chemical change, desciebe from a molecular viewpoint how diluting the solutions by adding water prior to mixing will decrease the rate of the reactionarrow_forwardConsider the attached energy diagram for the conversion of A → G. Question: Which points on the graph correspond to transition states?arrow_forward
- Which of the following is an application of the influence of temperature on chemical reaction rates? a. a solution is heated so more sugar can be dissolved in it b. food is put into a refrigerator to keep it from spoiling c. water is evaporated from a salt-water solution in order to recover solid salt d. more than one response is correctarrow_forwardThe following represents the effect of time on the concentration during a reaction. The upper line represents a reactant concentration and the lower line the concentration of a product. Which of the following statements is not correct? Concentration B C Time → O Between points A and D the reactant concentration is decreasing. O Between points B and C the product concentration is increasing. At point D the rate of the forward and reverse reactions are the same. O At point C the rate of product breakdown equals that of reactant consumption.arrow_forward1. Reactions that occur in the gaseous state are affected by changing the volume of the container. Which choice best describes what will happen to the reaction if the volume of the container is decreased? The reaction will shift right. The reaction will shift left. The reaction will not change. The reaction will speed up.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY