
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
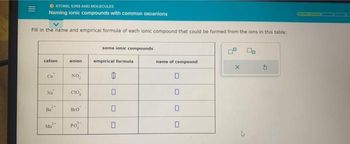
Transcribed Image Text:ATOMS, IONS AND MOLECULES
Naming ionic compounds with common oxoanions
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
cation
Cu
'2 "a
2+
Ba
3-
Ma
anion
NO,
CIO
Bro
PO
some ionic compounds
empirical formula
0
0
0
name of compound
0
0
0
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Iii (he Lewis structures listed here, M and X represent various elements iii the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element: (a) (b) (c) (d)arrow_forwardA representative element (X) forms an ion with a 2 charge. What is the a. Roman numeral group number for element X b. Lewis symbol for element X c. number of valence electrons possessed by X d. the chemical formula of the compound formed between X and the Ca2+ ionarrow_forwardOccasionally, we will see an ionic compound that has a 1 counterion. (Later we will find that 1 counterions are often more than spectators and take an active role in many reactions.) a. What elements (other than H) on the periodic table are most likely to form a 1 anion? b. Draw a Lewis structure for the ionic compound NH4Cl (Hint. One atom is a 1 counterion).arrow_forward
- Write the Lewis symbols for each of the following ions: (a) As3 (b) I (c) Be2+ (d) O2 (e) Ga3+ (f) Li+ (g) N3arrow_forwardWhich brand in each of the following pairs has the greater ionic character? msp;a.NaForNaIb.LiClorCsClc.CaSorC0d.MgNorMgParrow_forwardWrite a formula for each of the following compounds: (a) silicon dioxide (b) silicon tetraiodide (c) silane (d) silicon carbide (6) magnesium silicidearrow_forward
- For each of the following molecular models, write an appropriate Lewis formula.arrow_forwardEach of the following Lewis symbols represents a Period 3 element. Determine each elements identity.arrow_forwardFrom their positions in the periodic able, arrange the atoms in each of the following series in order of increasing electronegativity: (a) C, F, H, N. O (b) Br. Cl, F, H, I (c) F. H, O. P. S (d) AI, H. Na, O. P (e) Ba. H, N, O, Asarrow_forward
- Please help me completearrow_forwardWhich of the following is true about the Lewis structure of NF3? O 26 valence electrons have to be accounted for in NF3 O There are single covalent bonds between N and F O The N atom is less electronegative than the F and so occupies the central position in the skeletal structre O All of the above 目 DELL -> %24arrow_forwardPlease HELP me complete thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax