
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Reproduce the binary phase diagram depicted
in Fig. 3.20. For the composition shown at X, mark on
the diagram approximate temperatures used for the three
main thermal process steps used in precipitation hardening:
solution treatment, quenching, and aging.
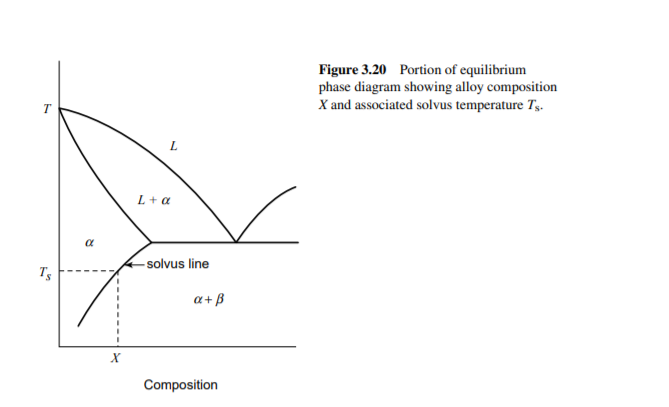
Transcribed Image Text:Figure 3.20 Portion of equilibrium
phase diagram showing alloy composition
X and associated solvus temperature Tș.
- solvus line
Ts
a+ B
х
Composition
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Solve correctly please,with some explanation needarrow_forward6) The y phase in steel can transform into Pearlite and through diffusional processes.arrow_forwardQuenching is a process used to preserve at room temperature a phase which exists in equilibrium at high temperature but which would decompose during equilibrium cooling. Explain the basis of this process in the precipitation process and martensitic transformation. Use phase diagrams when necessary.arrow_forward
- I need the answer as soon as possiblearrow_forwardDo not give answer in image and hand writingarrow_forwardCompute the volume percent of graphite VGr in a 3.3 wt% C cast iron, assuming that all the carbon exists as the graphite phase. Assume densities of 7.9 and 2.3 g/cm3 for ferrite and graphite, respectively. a. what is the weight fraction Wa? b. what is the weight fraction of graphite? c.what is the volume fraction of graphite?arrow_forward
- Using the isothermal transformation curve for a Eutectoid steel, determine the final structure of alloy for the following cooling processesarrow_forwardQ6/ A. Show how the precipitation heat treatment of precipitation hardenable alloys differs from dispersion hardening process of composite material. B. What are the main constitutes of composite materials and what are the roles of each constitute?arrow_forwardAccording to the following graph, two samples of 1080 steel are cooled from the eutectoid temperature, one at a cooling rate of 250°C/s and the other at a cooling rate of 7.27x10-8 °C/s. Specify the phases obtained and explain their formation from thermodynamic and kinetic perspectives. Also, briefly describe their formation. Draw the microstructure of the phases obtained. Sıcaklık (C) 800 700 600 500 400 300 200 100 0 10 1 T M(başlama) M(% 50) M(% 90) 10 M+O -Otektoid Sıcaklık 10² Zaman (s) % 50 10³ 104 105arrow_forward
- Referring to Figure 1, please answer the following [NOTE: SHOW YOUR WORK on Figure 1]:a. provide the specific name for the phase boundary lines denoted by A and B. b. identify the phases present at equilibrium in the phase fields denoted by C, D, and E. c. identify the specific name for the point denoted by F. d. What is the maximum solid solubility of Mg in Al? At what temperature does it occur? e. An Al-Mg alloy (10 wt% Mg, 90 wt% Al) is heated slowly (to insure equilibrium) from a temperature of 200 C: i. At what temperature does the first liquid phase form? ii. What is the composition of the liquid phase at the temperature in part i.? iii. At what temperature does complete melting (no solid phase remaining) occur? iv. What is the composition of the last solid remaining prior to complete melting? f. Using the equilibrium phase diagram of Figure 1, identify the phases present, their compositions, and their relative mass fractions at equilibrium for a 70 wt% Mg – 30 wt% Al…arrow_forwardGiven the equilibrium phase diagram (a) below, briefly describe the mechanisms of precipitation hardening/strengthening of an aluminium alloy making reference to the transitions shown in (b) below to X, X to A, X to D, A to B and A to C. а +0 D B Time (a) (b) - anedua Temperaturearrow_forwardPlease show all your work no ai. I'm trying to truly understand.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY