
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
question and answer attached.
could someone show clearer working and reasoning behind solotion
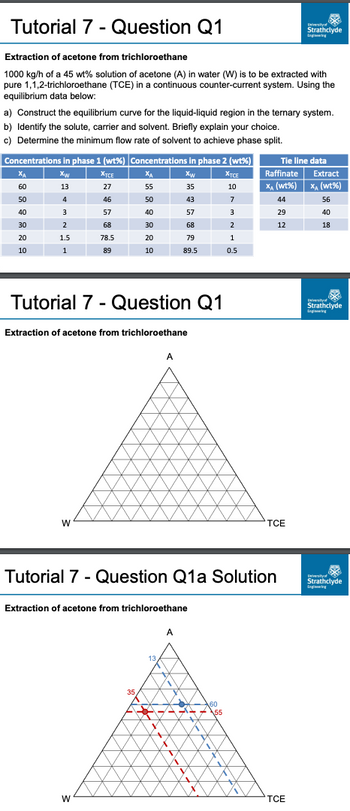
Transcribed Image Text:Tutorial 7 - Question Q1
Extraction of acetone from trichloroethane
1000 kg/h of a 45 wt% solution of acetone (A) in water (W) is to be extracted with
pure 1,1,2-trichloroethane (TCE) in a continuous counter-current system. Using the
equilibrium data below:
a) Construct the equilibrium curve for the liquid-liquid region in the ternary system.
b) Identify the solute, carrier and solvent. Briefly explain your choice.
c) Determine the minimum flow rate of solvent to achieve phase split.
Concentrations in phase 1 (wt%) Concentrations in phase 2 (wt%)
Xw
Xw
13
35
4
3
2
1.5
1
ХА
60
50
40
30
20
10
XTCE
27
46
57
68
78.5
89
W
Tutorial 7 - Question Q1
ХА
55
50
40
30
20
10
Extraction of acetone from trichloroethane
W
35
Extraction of acetone from trichloroethane
12
13.
A
30-
x
+
43
57
68
79
89.5
Tutorial 7 - Question Q1a Solution
A
X
A
V
4
K
X
60
A
55
XTCE
10
X
7
3
2
1
0.5
A
University of
Strathclyde
Engineering
Tie line data
Raffinate
Extract
X₁ (wt%) X₁ (wt%)
44
56
29
40
12
18
TCE
TCE
University of
Strathclyde
☆
University
Strathclyde
Engineering
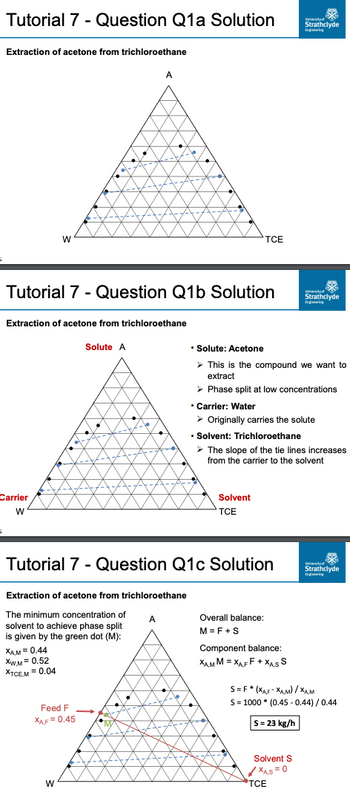
Transcribed Image Text:Tutorial 7 - Question Q1a Solution
Extraction of acetone from trichloroethane
W
Carrier
W
Tutorial 7 - Question Q1b Solution
Extraction of acetone from trichloroethane
d
4
W
Solute A
Feed F
XAF 0.45
.
.
Extraction of acetone from trichloroethane
The minimum concentration of
solvent to achieve phase split
is given by the green dot (M):
XAM = 0.44
XW.M= 0.52
XTCEM = 0.04
A
(.)
.
A
• Solute: Acetone
Tutorial 7 - Question Q1c Solution
TCE
. Solvent: Trichloroethane
This is the compound we want to
extract
> Phase split at low concentrations
• Carrier: Water
➤ Originally carries the solute
Solvent
TCE
The slope of the tie lines increases.
from the carrier to the solvent
Overall balance:
M=F+S
Component balance:
XAM M=XAFF+XAS S
Carathclyde
University of
University of
Strathclyde
Solvent S
✓✓XAS=0
TCE
S=F* (XAF-XAM)/XAM
S = 1000 (0.45-0.44) / 0.44
S = 23 kg/h
University of
Strathclyde
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
could you touch further on the last slide, where does the mixing point come from and also tie lines ?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
could you touch further on the last slide, where does the mixing point come from and also tie lines ?
Solution
by Bartleby Expert
Knowledge Booster
Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The