
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
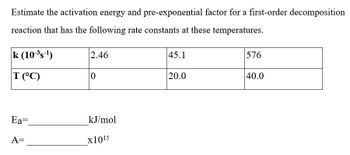
Transcribed Image Text:Estimate the activation energy and pre-exponential factor for a first-order decomposition
reaction that has the following rate constants at these temperatures.
k (10-³s-¹)
T (°C)
Ea=
A=
2.46
0
kJ/mol
x10¹5
45.1
20.0
576
40.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- Consider the reaction H2CO(g) + 02(g) Co2(g) + H20(1) for which AH° = -563.3 kJ and AS° = -140.5 J/K at 298.15 K. (1) Calculate the entropy change of the UNIVERSE when 2.085 moles of H,CO(g) react under standard conditions at 298.15 K. ASuniverse = J/K (2) Is this reaction reactant or product favored under standard conditions? (3) If the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored choose 'reactant favored'. Submit Answerarrow_forwardWhich of the following statements is TRUE regarding the spontaneity of the exothermic reaction listed below? 2 Na(s) + Clzlg) → 2 NaCi(s) The reaction is spontaneous as written only at high temperatures. O The reaction is spontaneous as written only at low termperatures. O There is not enough information provided to determine the spontaneity of the reaction. O The reaction is nonspontaneous as written at all temperatures. O The reaction is spontaneous as written at all temperatures. Question 19 Consider the following chemical reaction for the combustion of hydrogen gas: 2 H2lg) + Ozlg) 2 H2Olg) AHn = -483.6 kJ; ASan = -89.0 J/K Exn %3! What is the equilibrium constant for the reaction at 873.15 K? O 5.22 x 10-25 O 1.06 O 3.81 - 10 O 192 * 104 O 263 - 104arrow_forwardFor the following reaction 6 experiments have been run and the data collected is in the following table: 2 MnO4-(aq) + 5 H2C2O4 (aq) + 6 H+ (aq) ---> 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) Experiment [MnO4-], M [H2C2O4], M [H+], M Rate, M/s 1 0.2410 0.3470 0.2690 0.1147 2 0.3260 0.6210 0.2270 0.2776 3 0.5630 0.5740 0.7420 0.4431 4 0.2410 0.3470 0.3840 0.1147 5 0.4140 0.5740 0.5610 0.3258 6 0.3260 0.4930 0.4910 0.2203 a) Write the rate expression. _____________ b) Calculate the rate constant, k. __________________ c) If the concentration of MnO4-,H2C2O4, & H+ are 0.155 M, 0.293M, and 0.475M respectively, what is the rate of the reaction? ____________________arrow_forward
- Consider the reaction2POCl3 (g) → 2PCl3 (g) +O2 (g)a. Calculate ΔG° for this reaction. The ΔG°f values for POCl3(g) and PCl3(g) are -502 kJ/mol and -270. kJ/mol,respectively.b. Is this reaction spontaneous under standard conditions at 298 K?c. The value of 6S' for this reaction is 179 J/K mol .At what temperaturesis this reaction spontaneous at standard conditions? Assume that ΔH°and ΔS°' do not depend on temperature.arrow_forward2. Consider the following reaction at constant P. Determine the value of ASsurr at 298 K, then predict whether this reaction will be spontaneous at this temperature. N2(g) + 2 02(g) → 2 NO2(g) AH = +66.4 kJarrow_forwardUF6 is a compound used in the process of enriching uranium. Use the attached thermodynamic data to calculate the temperature for the following phase transition shown at 1 atm. UF6(s) → UF6(g) UF6(g) UF6(s) AH,(g), kJ/mol -2148 -2197 AG, (g), kJ/mol -2029 -2008 s'(g), J/K-mol 377 228 Select an answer and submit. for keyboard navigation, use the up/down arrow keys to select an answer. a 55.9°C b. 329°C 304°C 57.0'C e 62.3°Carrow_forward
- Consider the reaction2NO(g) + O2(g)2NO2(g)Using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15K.ANSWER: ΔGof (kJ/mol) NO: 86.6kj/mol NO2: 51.3 O2: 0arrow_forwardQUESTION 9 Regarding the following reaction at constant temperature, calculate the ratio of Kx at total pressure of 5.0 bar to that at 1.5 bar. Please enter your answer with 1 decimal. For example, 0.567 is written as 0.6. H₂CO (g) = CO (g) + H₂ (g).arrow_forwardProblem 2. With a particular catalyst and at a given temperature, the oxidation of naphthalene to phthalic anhydride proceeds as follows: R A 2 3 A = naphthalene R = naphthaquinone Sphthalic anhydride T = oxidation products k₁ = 0.21 S-¹ k₂ = 0.20 S-¹ K3 = 4.20 S-¹ k4= 0.004 s¹ ST What reactor type gives the maximum yield of phthalic anhydride? Roughly estimate this yield and the fractional conversion of naphthalene which will give this yield. (note: the word "roughly").arrow_forward
- 1. The vapor pressure of ethane between 100 K and 200 K is given by In P/MPa = 45.8006 - 2681.5/T + 0.012366T - 6.8688InT Calculate the normal boiling point of ethane A. 171.45 B. 184.53 C.201.44 D. 196.13 2. Consider a cell undergoing the reaction below at 298 K 2Na(s) + Cd2+ (aq) - - >> 2Na+ (aq) + Cd(s) When [Na+] = 0.0005 molal (γ = 0.974) and [Cd2+] = 0.2 molal (γ = 0.102), then Ecell = +2.45 V. What is E° for the cell.arrow_forward13.86. The rate constant for the reaction NO₂(g) + O3(g) → NO3(g) + O₂(g) was determined over a temperature range of 40 K, with the following results: T (K) 203 213 223 233 243 k (M¹s¹) 4.14 X 105 7.30 X 105 1.22 X 106 1.96 X 106 3.02 X 106 a. Determine the activation energy for the reaction. b. Calculate the rate constant of the reaction at 300 K.arrow_forward1. For the methanol synthesis reaction CO (g) + 2 H 2 (g) ↔ CH 3 OH (g) the equilibrium constant is given by: ln K = 11.988 + (9143.6 /T) − 7.492 ln T + 4.076×10−3T − 7.161×10−8T2 where T is in K. Estimate the standard heat of reaction at 473 K using the above relation. Book: Introduction to Engineering Thermodynamics 8 editionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The