
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
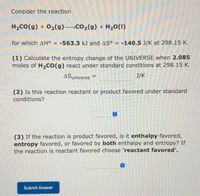
Transcribed Image Text:Consider the reaction
H2CO(g) + 02(g) Co2(g) + H20(1)
for which AH° = -563.3 kJ and AS° = -140.5 J/K at 298.15 K.
(1) Calculate the entropy change of the UNIVERSE when 2.085
moles of H,CO(g) react under standard conditions at 298.15 K.
ASuniverse =
J/K
(2) Is this reaction reactant or product favored under standard
conditions?
(3) If the reaction is product favored, is it enthalpy favored,
entropy favored, or favored by both enthalpy and entropy? If
the reaction is reactant favored choose 'reactant favored'.
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- How many moles of HNO₃ will be produced from the reaction of 27.5 g of NO₂ with excess water in the following chemical reaction? 3 NO₂(g) + H₂O (l) → 2 HNO₃(g) + NO(g)arrow_forwardOver the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is the following. 3 CoO(s) + 2 Al(s) → 3 Co(l) + Al2O3(s) What masses of cobalt(II) oxide and aluminum must be used to produce 12.9 g of cobalt? What is the maximum mass of aluminum oxide that could be produced?arrow_forwardConsider the following dissolution of lead(II) fluoride (PbF>(s): O 5.9 kJ molr1 PbF>(s) - Pb2*(ag) + 2 F(ag) O 5.9 kJ mol1 Below is a table of thermodynamic quantities pertaining to this reaction: Submit Request Answer Substance AHP (kJ mol1) S° (J K-1 mol1) Part B PBF2(s) -664.0 110.5 Determine the standard entropy of reaction (A,S°) for the dissolution of lead(II) fluoride. Pb2*(aq) 0.9 18.5 O 119.6 J K1 mol1 F(ag) -335.4 -13.8 O -105.8 J K1 mor1 O 119.6 J K1 mor1 O 105.8 J K-1 mol1arrow_forward
- Consider the following reaction: 10 KNO3(s) +8 C(s) +3 S(s) 2 K2CO3(s) + 3 K,SO,(s) + 6 CO2(g) + 5 N2(g) you mix 0.75 kg of KNO3 with 0.75 kg of sulfur in the presence of excess carbon and the reaction proceeds with 55% yield, what mass of gas-phase products (i.e., CO2 plus N2) will leave the reaction mixture? Show complete work including all calculations, units, appropriate significant figures, and 1-5 key words of explanation at each step of your calculation. Ifarrow_forwardFor the following reaction at equilibrium in a reaction vessel, which one of the changes below would cause the Br2 concentration to decrease? 2NOBr(g) ↔ 2NO(g) + Br2(g) ΔH= 30 kJ/mol Increase the temperature Remove some NO Add more NOBr Compress the gas mixture into a smaller volumearrow_forwardConsider the reaction: 2BRF3(g)Br,(g) + 3F2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.06 moles of BrF3(g) react at standard conditions. AS°. system J/Karrow_forward
- Given the following reaction and using the data in the table, determine the order of the reaction:2NO2(g)→2NO(g)+O2(g) Time (sec) [NO2 ] ln [NO2 ] 1/[NO2 ] 0 0.0100 -4.605 100 100 0.00648 -5.039 154 200 0.00479 -5.341 209 300 0.00380 -5.573 263 400 0.00315 -5.760 317 500 0.00269 -5.918 372 600 0.00235 -6.057 426 a. none of these b. second c. zero d. third e. firstarrow_forwardThe growth of baker's yeast (Saccharomyces cerevisiae) on glucose under anaerobic conditions can be described by the following equation:C6H12O6 + β NH3 → 0.59 CH1.74N0.2O0.45 + 0.43 C3H8O3 + 1.54 CO2 + 1.3 C2H5OH + 0.036 H2O a) Determine the value of the biomass yield coefficient YX/Sb) Determine the values of the yield coefficients in products YEtOH/S and Yglycerol/Sc) Calculate the value of the coefficient βarrow_forwardConsider the following reaction in a sealed vessel kept at constant temperature: A(g) ⇌ 2B(g) If the reaction is started with 2.0 mol of A and no B, the amount of B at equilibrium is 3.0 mol. How many moles of A should one start with to obtain 6.0 mol of B at equilibrium under the same conditions (same vessel, same temperature, no gas B present initially)? A. 5.6 mol B. 5.0 mol C. 4.0 mol D. 6.0 mol E. 6.5 molarrow_forward
- 1. Cyclohexane can be produced from the hydrogenation of benzene according to the reaction C6H6 (g) + 3 H2 (g) --> C6H12 (g) Ka = 1.371 at 550 K The reactor temperature is 550K and hydrogen to benzene feed mole ratio is 4.5:1. Estimate the gas composition under equilibrium conditions if the reactor pressure is 30 bar. The following fugacity coefficients are obtained for the species involved: Fugacity coefficient (550K, 30 bar) Cyclohexane 0.907 Benzene 0.919 Hydrogen 1.003arrow_forwardThe equilibrium constant for the reaction of nitrogen and hydrogen to give ammonia is 0.118 at 745 K. The balanced equilibrium equation is as follows: N2(g) + 3H₂(g) = 2NH3(g) What is Kp for this reaction at the same temperature? (R: 0.082 L atm/mol.K)arrow_forwardHow many electrons are transferred in the following process, given the unbalanced reaction? PbO2 (s) + H* (aq) + Fe (s) → Fe3+(aq) + Pb2+ (aq) + H20 (1) Group of answer choices A-1 В-2 C-6 D-4 Е-3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The