
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
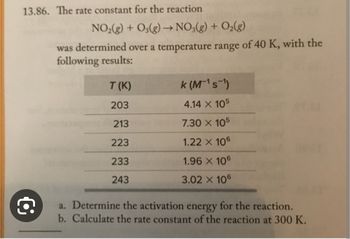
Transcribed Image Text:13.86. The rate constant for the reaction
NO₂(g) + O3(g) → NO3(g) + O₂(g)
was determined over a temperature range of 40 K, with the
following results:
T (K)
203
213
223
233
243
k (M¹s¹)
4.14 X 105
7.30 X 105
1.22 X 106
1.96 X 106
3.02 X 106
a. Determine the activation energy for the reaction.
b. Calculate the rate constant of the reaction at 300 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Consider the following dissolution of lead(II) fluoride (PbF>(s): O 5.9 kJ molr1 PbF>(s) - Pb2*(ag) + 2 F(ag) O 5.9 kJ mol1 Below is a table of thermodynamic quantities pertaining to this reaction: Submit Request Answer Substance AHP (kJ mol1) S° (J K-1 mol1) Part B PBF2(s) -664.0 110.5 Determine the standard entropy of reaction (A,S°) for the dissolution of lead(II) fluoride. Pb2*(aq) 0.9 18.5 O 119.6 J K1 mol1 F(ag) -335.4 -13.8 O -105.8 J K1 mor1 O 119.6 J K1 mor1 O 105.8 J K-1 mol1arrow_forwardConsider the following reaction: 10 KNO3(s) +8 C(s) +3 S(s) 2 K2CO3(s) + 3 K,SO,(s) + 6 CO2(g) + 5 N2(g) you mix 0.75 kg of KNO3 with 0.75 kg of sulfur in the presence of excess carbon and the reaction proceeds with 55% yield, what mass of gas-phase products (i.e., CO2 plus N2) will leave the reaction mixture? Show complete work including all calculations, units, appropriate significant figures, and 1-5 key words of explanation at each step of your calculation. Ifarrow_forwardGiven the following reaction and using the data in the table, determine the order of the reaction:2NO2(g)→2NO(g)+O2(g) Time (sec) [NO2 ] ln [NO2 ] 1/[NO2 ] 0 0.0100 -4.605 100 100 0.00648 -5.039 154 200 0.00479 -5.341 209 300 0.00380 -5.573 263 400 0.00315 -5.760 317 500 0.00269 -5.918 372 600 0.00235 -6.057 426 a. none of these b. second c. zero d. third e. firstarrow_forward
- The growth of baker's yeast (Saccharomyces cerevisiae) on glucose under anaerobic conditions can be described by the following equation:C6H12O6 + β NH3 → 0.59 CH1.74N0.2O0.45 + 0.43 C3H8O3 + 1.54 CO2 + 1.3 C2H5OH + 0.036 H2O a) Determine the value of the biomass yield coefficient YX/Sb) Determine the values of the yield coefficients in products YEtOH/S and Yglycerol/Sc) Calculate the value of the coefficient βarrow_forward1. An impure sample of compound A is contaminated with two impurities B and C. The sample is to be purified by recrystallization using ethanol as the solvent. The solubility properties of the three components are summarized below. Solubility in Solubility in ethanol Solubility in 50 mL Solubility in 50 mL ethanol at -78 °C at - 0°C ethanol at -78 °C ethanol at - 0°C (g) (g) Compound A 0.12 g/mL 0.02 g/mL Impurity B 0.58 g/mL 0.04 g/mL Impurity C 0.005 g/mL 0.0003 g/mL The impure (7.5 g) sample contains 5.0 g of compound A, 1.5 g of B and 1.0 g of C and is recrystallized using 50 mL of ethanol. The sample is boiled with 50 mL of ethanol, filtered by gravity and then cooled in ice and filtered by suction. a) How much compound A should be obtained as the final product? Will the sample be contaminated with any of the impurities? Explain (using calculations to support your answer-fill in the missing masses in the table above). Hint: For this question you should calculate the mass of each…arrow_forwardConsider the following reaction in a sealed vessel kept at constant temperature: A(g) ⇌ 2B(g) If the reaction is started with 2.0 mol of A and no B, the amount of B at equilibrium is 3.0 mol. How many moles of A should one start with to obtain 6.0 mol of B at equilibrium under the same conditions (same vessel, same temperature, no gas B present initially)? A. 5.6 mol B. 5.0 mol C. 4.0 mol D. 6.0 mol E. 6.5 molarrow_forward
- A student determines the value of the equilibrium constant to be 1.98×10-5 for the following reaction.CO2(g) + H2(g)CO(g) + H2O(g)Based on this value of Keq:G° for this reaction is expected to be (greater, less) fill in the blank 1 than zero.Calculate the free energy change for the reaction of 1.74 moles of CO2(g) at standard conditions at 298K. G°rxn = kJarrow_forwardHow many electrons are transferred in the following process, given the unbalanced reaction? PbO2 (s) + H* (aq) + Fe (s) → Fe3+(aq) + Pb2+ (aq) + H20 (1) Group of answer choices A-1 В-2 C-6 D-4 Е-3arrow_forwardThe equilibrium constant, K., for the following reaction is 9.52×10-2 at 350 K. CH4 (g) + CC14 (g) =2 CH2Cl2 (g) Calculate the equilibrium concentrations of reactants and product when 0.277 moles of CH, and 0.277 moles of CCl, are introduced into a 1.00 L vessel at 350 K. [ CH4] M [CC4] M [ CH2C1, ] = Marrow_forward
- Part a plz!arrow_forwardNitromethane, CH3NO2 , can be used as a fuel. When the liquid is burned, the (unbalanced) reaction is mainlyCH3NO2(l) + O2(g) → CO2(g) + N2(g) + H2O(g)a. The standard enthalpy change of reaction (ΔH°van ) for the balanced reaction (with lowest whole-number coefficients) is −1288.5 kJ. Calculate ΔHf0 for nitromethane.b. A 15.0-L flask containing a sample of nitromethane is filled with O2 and the flask is heated to 100.°C. At this temperature, and after the reaction is complete, the total pressure of all the gases inside the flask is 950. torr. If the mole fraction of nitrogen (χnitrogen) is 0.134 after the reaction is complete, what mass of nitrogen was produced?arrow_forwardQ3 in imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The