
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
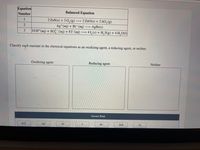
Transcribed Image Text:Equation
Number
Balanced Equation
1
2 ZnS(s) + 3 O,(g)
2 ZnO(s) + 2 S0,(g)
Ag*(aq) + Br¯(aq)
10 H*(aq) + SO (aq) + 8 I¬(aq)
→ AgBr(s)
3
4 L, (s) + H, S(g) + 4 H, O(1)
Classify each reactant in the chemical equations as an oxidizing agent, a reducing agent, or neither.
Oxidizing agent
Reducing agent
Neither
Answer Bank
SO
Ag*
Br
ZnS
H+
Transcribed Image Text:<>
so?-
Ag*
H+
I-
Br
ZnS
O2
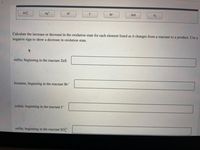
Calculate the increase or decrease in the oxidation state for each element listed as it changes from a reactant to a product. Use a
negative sign to show a decrease in oxidation state.
sulfur, beginning in the reactant ZnS
bromine, beginning in the reactant Br
iodine, beginning in the reactant I-
sulfur, beginning in the reactant SO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Moving to another question will save this response. Question 2 What is the net ionic equation for the reaction of aqueous potassium hydroxide and aqueous magnesium chlori 1S K (aq) + OH (aq) KOH(s) КОНЕ) K*(aq) + Cl (aq) →KCI(s) Mg"(aq) + 2 CF (aq) MgCl2(s) 2+ o Mg-(aq)+3 OH (aq)Mg(0H)3(s) Mg-(aq) -2 OH (aq) Mg(OH)2(s) Me (aq) - Он (аq) — MgОН (9) MGOH (s A Moving to another question will save this response. search hp f11 f12 fg f10 f6 f7 f8 f4 f5 I미 & %24 4. 6. 7. 80 T. | 0Oarrow_forwardGiven the following unbalanced equation, select the correct net ionic equation. CaCO3(s) + HCI(aq) CaCl2(aq) + H20(1) + CO2(g) --> (A) Ca2*(aq) + 2CI^(aq) CaCl2(aq) --> (B) CO32-(aq) + 2H*(aq) --> H20(1) + CO2(g) (C) CaCO3(s) + 2H*(aq) + 2CI¯(aq) CaCl2(aq) + H20(1) + CO2(g) --> (D) CaCO3(s) + 2H*(aq) --> Ca2*(aq) + H20(1) + CO2(g)arrow_forwardWhat is the balanced net ionic equation for the reaction between aqueous solutions of acetic acid and sodium hydroxide? CH3CO₂H(aq) + OH (aq)-CH3 (aq) + CO2(g) + H₂O(0) Ob CH3CO₂H(aq) + 2 Na* (aq) + 2 OH (aq) → CH3CO₂2 (aq) + 2Na* (aq) + H₂O(0) OCH3CO₂H(aq) + 3 NaOH(aq)-CCO₂H³ (aq) + 3 Na* (aq) + H₂O(0) OCH3CO₂H(aq) + OH (aq) → CH3(aq) + CO3²- (aq) + H₂(g) OCH3CO₂H(aq) + OH (aq)-CH3CO2 (aq) + H₂O(l)arrow_forward
- Balance the following reaction. If a reactant or product is not present, put a zero (0) in the blank. H+(aq) + H2O(l) + ClO-13(aq) + e-1 <--> Cl-1(g) + H+(aq) + H2O(l)arrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO, (s) + 4 HCI(aq) MnCl, (aq) + 2 H,0(1) + Cl, (g) How much MnO, (s) should be added to excess HCl(aq) to obtain 205 mL Cl, (g) at 25 °C and 805 Torr? mass of MnO,:arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → ɖ(C103) (aq) + H₂O(1) 0-0 Śarrow_forward
- Please don't provide handwritten solutionarrow_forward5. Identify each of the following reactions as either synthesis, decomposition, single displacement, double displacement, combustion, or neutralization 2Li(s) + H₂O(1)→ Li₂O(s) + H2(g) SO2(g) + H₂O → H₂SO3(aq) 02|| AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) + 2NaOH(aq) → Na₂CO3(aq) + H₂CO3(aq) 2H₂O C3H8(g) + 502(g) → 3CO2(g) + 4H₂O(1) Mg(OH)2(aq) → MgO(s) + H₂O(1)arrow_forwardCould you please balance each equation and tell me what type of equation it isarrow_forward
- When an ionic solid dissolves in water, water molecules attract the ions causing them to dissociate orcome apart. The resulting dissolved ions are electrically charged particles that allow the solution toconduct electricity. The following chemical equations represent this phenomenon:NaCl (s) → Na+ (aq) + Cl-(aq)Na2CO3 (s) → 2Na+(aq) + CO32- (aq)Write a similar balanced chemical equation for the other four strong electrolytes in the data table NaHCO3 KNO3NH4Cl HClarrow_forwardConsider the reaction of iron (III) chloride and sodium hydroxide to form iron (III) hydroxide and sodium chloride. Which of the following depicts the balanced, total ionic equation? Fe3+ (aq) + 3OH- (aq) --> Fe(OH)3 (s) Fe3+ (aq) + 3Cl- (aq) + 3Na+ (aq) + 3OH- (aq) --> Fe3+ (s) 3(OH-) (s) + 3Na+ (aq) + 3Cl- (aq) Fe3+ (aq) + 3Cl- (aq) + 3Na+ (aq) + 3OH- (aq) --> Fe(OH)3 (s) + 3Na+ (aq) + 3Cl- (aq) Fe3+ (aq) + 3Cl- (aq) + Na+ (aq) + OH- (aq) --> Fe(OH)3 (s) + Na+ (aq) + Cl- (aq)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY