
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
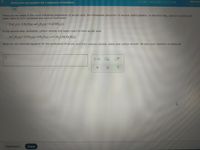
Transcribed Image Text:Writing the net equation for a sequence of reactions
0/5
ARLYQUI
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and
water react to form acetylene and calcium hydroxide:
CaC,(s)+2 H,O(g)-C,H,(g)+Ca(OH),(s)
In the second step, acetylene, carbon dioxide and water react to form acrylic acid:
6 C,H,(g)+3 CO,(g)+4H,O(g)→5 CH,CHCO,H(g)
Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.
C-C
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The complete combustion of gasoline (C8H18) is best represented by which of the following equations Options: 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O(l) 2 C8H18 (l) + 17 O2 (g) → 16 CO (g) + 9 H2O (l) C8H18 (l) + O(g) → 8 CO2 (g) + 9 H2O (l) C8H18 (l) → 8 C (s) + 9 H2 (g)arrow_forwardThe compound sodium thiosulfate pentahydrate, Na2S2 O3-5H2O, is important commercially to the photography business as "hypo," because it has the ability to dissolve unreacted silver salts from photographic film during development. Sodium thiosulfate pentahydrate can be produced by boiling elemental sulfur in an aqueous solution of sodium sulfite. S8 (s) + Na2 SO3 (aq) + H2O(1) → Na2 S2 O3-5H2 O(s) (unbalanced) What is the theoretical yield of sodium thiosulfate pentahydrate when 3.25 g of sulfur is boiled with 13.1 g of sodium sulfite? Sodium thiosulfate pentahydrate is very soluble in water. What is the percent yield of the synthesis if a student doing this experiment is able to isolate (collect) only 5.26 g of the product?arrow_forward7:25 ◄ Search 2- 3c₂²- 1 + Write a balanced chemical equation based on the following description: solid barium carbonate decomposes into solid barium oxide and carbon dioxide gas when heated BaCO3 (s) (s) Be O Question 5 of 20 Reset 2 3 4 5 6 7 8 B (1) Ox Ba Cb (g) C 个 Br Tap here or pull up for additional resources 5G 85 Ca Submit 9 11 (aq) O Cr • x H₂Oarrow_forward
- Dilution Problems, Chemistry, Molarity & Concentration Examples,... M O O CHEMICAL REACTIONS Reaction sequence stoichiometry In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: Fe₂O3(s) + 3CO(g) → 2Fe(s) + 3 CO₂ (g) E crosoft O V Blast furnaces extra pure iron from the iron (III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2C(s) + O₂(g) 2 CO (g) W Suppose the yield of the first step is 84.% and the yield of the second step is 63.%. Calculate the mass of oxygen required to make 3.0 kg of iron. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits. G Microsoft Microsoft 6.52.210... Explanation BO C Check 9,269 x10 믐 103 X OCT 24 ロ・ロ átv 2 3/5 DOZA Simrat 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility W E ol Ararrow_forwardesc Calculate the quantity of O, would be required to generate 13.0 mol of NO in the reaction below assuming the reaction has only 72.4% yield. 2 NO (g) + O₂(g) → 2 NO₂ (g) ! 1 S Q A O @ 2 W S CE #3 E D $ 4 R % 5 F LL T MacBook Pro ^ 6 G Y & 7 FEB 21 H zoom U 8 W -arrow_forwardSuppose the following chemical reaction can take place in this mixturearrow_forward
- Write a balanced chemical equation when solid barium carbonate decomposes into solid barium axide and carbon diaxide gas when heated. Choose the correct answer. O 2Bag(CO)2 (s) -> 2 BagO2 (s) + 2 CO2@) Ba (s)+ 2 CO3 (g) --> BaO2 (s) +2CO2 (s) O Baco3(s)--> Bao (s) + CO2 (e) O 4 Ba(CO2)3 (s) ->2 Baz0 (s) + 3C02@arrow_forwardWhat is true for this chemical reaction: CO₂(g) + 4H₂(g) = CH₂(g) + 2H₂O(g)? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b C Reaction Changes Homework Unanswered Due Today, 11:59 PM d AHrxn > 0; ASrxn > 0 AHxn 0 AHxn 0; ASxn < 0 Unanswered. 2 attempts leftarrow_forwardThere are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC,(s)+2H,O(g)–C,H,(g)+Ca(OH),(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6 C,H,(g)+3 CO,(9)+4H,0(9)→5 CH,CHCO,H(9) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced. Explanation Check O 2022 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center Accessibility 2:24 PM W 4/8/2022 DELL Esc F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 PrtScr Insert Delete PgUp PgDn Fn C@ 23 2$ Num Lock & Backspace 1 2 3 4 7 8. Co (Oarrow_forward
- Hi I need help writing out a balanced chemical equation.arrow_forwardWhen an ionic solid dissolves in water, water molecules attract the ions causing them to dissociate orcome apart. The resulting dissolved ions are electrically charged particles that allow the solution toconduct electricity. The following chemical equations represent this phenomenon:NaCl (s) → Na+ (aq) + Cl-(aq)Na2CO3 (s) → 2Na+(aq) + CO32- (aq)Write a similar balanced chemical equation for the other four strong electrolytes in the data table NaHCO3 KNO3NH4Cl HClarrow_forwardKindly refer to the attached picturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY