
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
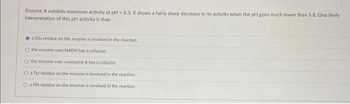
Transcribed Image Text:Enzyme X exhibits maximum activity at pH- 6.3. X shows a fairly sharp decrease in its activity when the pH goes much lower than 5.8. One likely
interpretation of this pH activity is that:
a Glu residue on the enzyme is involved in the reaction.
O the enzyme uses NADH has a cofactor
the enzyme uses coenzyme A has a cofactor
O a Tyr residue on the enzyme is involved in the reaction.
O a His residue on the enzyme is involved in the reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Km value should be more than 10% apart, use non-competitive inhibitorarrow_forwardSelect the incorrect statement. With regards to free energy ΔG of the reaction below E+S ⇌ ES Negative ΔG mean the reaction toward is facourable More negative value of ΔG indicates stronger binding to S to E It is possible to compute disassociation constant from the ΔG value alone It is possible to calculate the term ( ΔH – T ΔS) from the value of ΔGalone ΔG = 0 indicates (ES)/(E)(S) =1 None of the abovearrow_forwardNonearrow_forward
- Amino acids are linked through peptide bonds to form proteins. This linkage of amino acids in the formation of proteins accomplished by [ Select ] reaction V [ Select ] Hydrolysis Fission Condensation Fusionarrow_forward15) The graph at right shows the results of reaction rate vs. substrate concentration for a Michaelis-Menten type enzyme 16) Th a. True b. False* reaction rate substrate concentrationarrow_forwardWith a ∆G°´ of -16.7 kJ/mol, the reaction catalyzed by hexokinase is considered to be _____. at equilibrium substrate and product concentration dependent freely reversible metabolically irreversible none of the abovearrow_forward
- Bypass reaction in the anabolic pathway often occurs when A The ΔG of the reverse reaction in the catabolic pathway has a large negative value B The ΔG of the reverse reaction in the catabolic pathway has a large positive value C The ΔG of the reaction in the anabolic pathway is close to zero D The ΔG of the reaction in the anabolic pathway is positive E None of the abovearrow_forwardplease help me identify these huhu thank youarrow_forwardWhat kind of catalysis is cysteine driving in this picture? S Los i fy R……—NHR N-H---S O. N-H H-N H-N. O Metal catalysis Covalent catalysis Base catalysis O Acid catalysis NHR²arrow_forward
- 38. The shown reaction is one of the four repeating steps during fatty acid biosynthesis. Which of the following statements is correct? 유 CH3-C-CH₂-C-S-ACP A B OH 유 CH3-C-CH₂-C-5-ACP → A. The small molecule in box A is NADPH + H* B. It is the second reduction reaction during fatty acid biosynthesis C. Both A and B D. Neither A nor Barrow_forward1. The concentration of substrate X is high. What happens to the rate of the enzyme-catalyzed reaction if the concentration of substrate X is reduced? Explain. 2. An enzyme has an optimum pH of 7.2. What is most likely to happen to the activity of the enzyme if the pH drops to 6.2? Explainarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON