
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
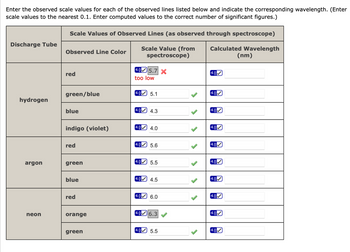
Transcribed Image Text:Enter the observed scale values for each of the observed lines listed below and indicate the corresponding wavelength. (Enter
scale values to the nearest 0.1. Enter computed values to the correct number of significant figures.)
Scale Values of Observed Lines (as observed through spectroscope)
Discharge Tube
Observed Line Color
Scale Value (from
spectroscope)
4.05.7 X
Calculated Wavelength
(nm)
4.0
red
green/blue
hydrogen
blue
too low
4.05.1
4.0
4.0
4.0
4.3
4.0
4.0
indigo (violet)
4.0
red
4.0
5.6
4.0
4.0
4.0
argon
green
5.5
blue
4.0 4.5
4.0
red
4.0 6.0
4.0
4.0
neon
orange
6.3
4.0
green
4.0
5.5
4.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 9 images

Knowledge Booster
Similar questions
- Draw, label, and explain the functions of the parts of a spectroscope.arrow_forwardConvert a a wavelength of 218A to cm1, b a frequency of 8.0771013s1 to cm1, c a wavelength of 3.31m to cm1.arrow_forwardThe maximum in the blackbody radiation intensity curve moves to shorter wavelength as temperature increases. The German physicist Wilhelm Wien demonstrated the relation to be max1/T . Later, Planck’s equation showed the maximum to be max=0.20hc/kt . In 1965, scientists researching problems in telecommunication discovered “background radiation” with maximum wavelength 1.05 mm (microwave region of the EM spectrum) throughout space. Estimate the temperature of space.arrow_forward
- What is the threshold frequency for sodium metal if a photon with frequency 6.661014s1 ejects an electron with 7.741020 J kinetic energy? Will the photoelectric effect be observed if sodium is exposed to orange light?arrow_forward6.10 Define the term refraction.arrow_forward6.9 If a string of decorative lights includes bulbs with wave-lengths of 480, 580, and 700 mm, what are the frequencies of the lights? Use Figure 6.6 to determine which colors are in the set.arrow_forward
- In a FranckHertz experiment on hydrogen atoms, the first two excitation thresholds occur at 10.1 and 11.9 eV. Three optical emission lines are associated with these levels. Sketch an energy-level diagram for hydrogen atoms based on this information. Identify the three transitions associated with these emission lines. Calculate the wavelength of each emitted line.arrow_forwardState how many radial, angular, and total nodes are in each of the following hydrogen-like wavefunctions. a 2s b 3s c 3p d 4f e 6g f 7sarrow_forwardThere are an infinite number of allowed electronic transitions in the hydrogen atom. Why dont we see more lines in the hydrogen emission spectrum?arrow_forward
- What is a rotating frame of reference?arrow_forwardA particular transition of the rubidium atom emits light whose frequency is 3.84 1014 Hz. (Hz is the abbreviation for hertz, which is equivalent to the unit/s, or s1.) Is this light in the visible spectrum? If so, what is the color of the light? (See Figure 7.5.)arrow_forwardUsing Table 5.2, write down the mathematical expression for the 2px wave function for an electronically excited H atom. Estimate the probability of finding the 2px electron if you look in a cubical box of volume of 0.8(pm)3 centered at a distance of 0.5001010m in the =/2 , =0 direction. Does this probability change as you change ? At what angles is the probability of finding the electron smallest and at what angles is the probability the largest? (Note that =2 is the same location as =0 , so don’t double count.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning