
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
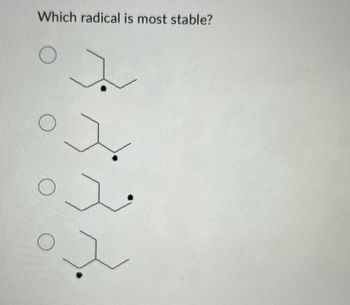
Transcribed Image Text:О
Which radical is most stable?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which radical is least stable?arrow_forward3. For the following structures: CH3a C-CH,CH3 C Hy' H (a) Determine whether each radical is a primary, secondary, or tertiary radical. (b) Rank these radicals in terms of their relative stability (1 = most stable to 3 = least stable). 4 Draw the (2) banoggata 1oarrow_forwardRank the following compounds from least to most stable. Least stable Most stablearrow_forward
- NBS heat Draw the molecule(s) on the canvas by choosing buttons from the Tools (for bonds and charges), A toolbars. H± 12D EXP. CONT. H C N O S F CI Br Br P F × Incorrect; Try Again; 2 attempts remaining Note that, in the second propagation step, bromine is attacked by the least sterically hindered carbon ra Δ A. Submit Previous Answers Request Answerarrow_forwardWhat is the predicted product for the reaction shown? O O O O O IV V OH an H IV || НО. N CrO3 CI H CH₂Cl₂ OH V CI |||arrow_forwardWhat is the resulting product and configuration?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning