
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
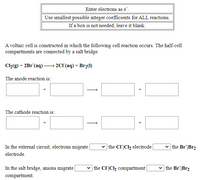
Transcribed Image Text:Enter electrons as e".
Use smallest possible integer coefficients for ALL reactions.
a box is not needed, leave it blank.
A voltaic cell is constructed in which the following cell reaction occurs. The half-cell
compartments are connected by a salt bridge.
Cl2(g) + 2Br (aq):
2C1(aq) + Br2(1)
The anode reaction is:
The cathode reaction is:
In the external circuit, electrons migrate
v the Cl|Cl2 electrode|
v the Br|Br2
electrode.
In the salt bridge, anions migrate
v the CI|Cl2 compartment
v the Br|Br2
compartment.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A voltaic cell is constructed in which the anode is a Cu* Cu** half cell and the cathode is a F F2 half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate ) the Cu"|Cu²* electrode the FF2 electrode. In the salt bridge, anions migrate | the Cu Cu" compartment| the FF2 compartment. from toarrow_forwardA voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a salt bridge. Pb²+ (aq) + Mg(s) Pb(s) + Mg2+ (aq) The anode reaction is: + The cathode reaction is: + 1 -0 In the external circuit, electrons migrate the Pb|Pb²+ electrode. In the salt bridge, anions migrate [ the Pb/Pb2+ compartment. + + the Mg|Mg2+ electrode the Mg|Mg2+ compartmentarrow_forwardEnter electrons as e". A voltaic cell is constructed in which the anode is a Ag Ag" half cell and the cathode is a Hg Hg* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coeficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migratel the AgAg" electrode v the Hg Hg* electrode. In the salt bridge, anions migrate| v the Hg|Hg" compartment| v the AgAg" compartment. +arrow_forward
- Write the overall reaction for the Redox reaction (below) when it occurs in a basic environment. H3ASO4 (aq) + Zn (s) → AsH3 (1) + Zn²+ (aq) Steps for writing half-cell reactions and overall Redox reactions: 1. Balance atoms other than H and O by changing their coefficients 2. Balance the O's and H's by adding OH and/or H₂O 3. Add electrons to balance the charge 4. Add the Oxidation and Reduction equations once the electrons in each cancel out 5. Cancel out (or reduce to the lowest possible numbers) any other species that are present on both sides of the reaction More Information H3ASO4 + AsH3 Zinc + Zinc ionsarrow_forwardEnter electrons as e-. Use smallest possible integer coefficients for ALL reactions. If a box is not needed, leave it blank. A voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a salt bridge.3Br2(l) + 2Al(s) ----> 6Br-(aq) + 2Al3+(aq)The anode reaction is: + + The cathode reaction is: + + In the external circuit, electrons migrate _(from or to?)__ the Al | Al3+ electrode _(from or to?) the Br- | Br2 electrode.In the salt bridge, anions migrate _(from or to?) the Br- | Br2 compartment _(from or to?) the Al | Al3+ compartment.arrow_forwardA voltaic cell is constructed in which the anode is a Pb|Pb2+ half cell and the cathode is a I-|I2 half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The net cell reaction is: + + In the external circuit, electrons migrate _(from or to?)__ the I-|I2 electrode _(from or to?)__the Pb|Pb2+ electrode.In the salt bridge, anions migrate _(from or to?)__ the Pb|Pb2+ compartment _(from or to?)__ the I-|I2 compartment.arrow_forward
- A voltaic cell is constructed in which the anode is a Ni|Ni2+ half cell and the cathode is a Hg|Hg2+ half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The net cell reaction is: + + In the external circuit, electrons migrate the Ni|Ni2+ electrode the Hg|Hg2+ electrode.In the salt bridge, anions migrate the Hg|Hg2+ compartment the Ni|Ni2+ compartment.arrow_forwardA voltaic cell is constructed in which the anode is a Cd|Cd2+ half cell and the cathode is a AgAg* half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: + The net cell reaction is: In the external circuit, electrons migrate the Ag|Ag* electrode v the Cd|Cd2+ electrode. In the salt bridge, anions migrate v the Ag|Ag" compartment v the Cd|Cd2+ compartment. from toarrow_forwardA voltaic cell is constructed from a standard = Sn2+ Sn half cell (E° red -0.140V) and a standard F2 F half cell (E° red = 2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. ↑ + + +arrow_forward
- Enter electrons as e A voltaic cell is constructed in which the anode is a Fe2+|Fe3+ half cell and the cathode is a F|F, half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: +. In the external circuit, electrons migrate the Fe2+|Fe3+ electrode the F|F, electrode. from to In the salt bridge, anions migrate the F|F, compartment 3- the Fe2+|Fe+ compartment. +. +. 1arrow_forwardA voltaic cell is constructed from a standard Hg2* Hg half cell (E°red = 0.855V) and a standard Br2 Br half cell (E°red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V.arrow_forwardEnter electrons as e¯. Use smallest possible integer coefficients for ALL reactions. If a box is not needed, leave it blank. A voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a salt bridge. Cl2(g) + 2Br (aq) → 2CI(aq) + Br2(1) The anode reaction is: + The cathode reaction is: + In the external circuit, electrons migrate the Cl|Cl, electrode v the Br|Br, electrode. In the salt bridge, anions migrate v the Cl|Cl2 compartment v the Br|Br2 compartment. 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY