
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The formula for nitryl chloride is CINO2 (in which N is the central atom).
a.Draw the Lewis structure for the molecule, including all resonance structures.
b.What is the N-O bond order?
c.Describe the electron-pair and molecular geometries and give values for all bond angles.
d.What is the most polar bond in the molecule? Is the molecule polar?
e.The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule: A =-0.03, B = -0.26, and C = +0.56. Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?
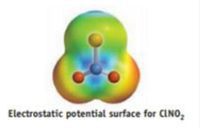
Transcribed Image Text:Electrostatic potential surface for CINO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- put each in the appropriate spacearrow_forwardThis sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't ze of the molecule be? possible chemical formula (check all that apply) electrostatic potential map Oco, O COCI, O H,0 O SO, O OCS I Don't Know Submit ill LIC AIL Rightarrow_forwardLewis dot structure for HNOarrow_forward
- The measure of the attraction of an atom of a given element, in a molecule, for the electrons in a covalent bond to that atom is called the of that element.arrow_forward7.72 How does an MSN differ from amorphous silica so that is has improved biocompatibility?arrow_forward7.13 Figure 7-2 depicts the interactions of an ion with its first nearest neighbors, second nearest neighbors, and third nearest neighbors in a lattice, (a) Would the interactions with the fourth nearest neighbors be attractive or repulsive? (b) Based on Coulomb's law, how would the relative sizes of the terms compare if the potential energy were expressed as V=V1st+V2nd+V3rd+V4th ?arrow_forward
- Give detailed Solution with explanation needed. Avoid handwritten Solution. Don't give Ai generated solutionarrow_forwardI need 23 and 24arrow_forwardLennard-Jones potential diagrams, also called intermolecular potential energy diagrams, illustrate the relationship between the potential energy of a molecule as the distance between the two nuclei changes. Select all of the true statements regarding Lennard-Jones potential diagrams.1. As the internuclear distance decreases, the attraction between the nuclei increases significantly, causing a steep increase in the potential energy of the system.2. Once a balance is found between the attractive and repulsive forces, the outer orbitals are able to overlap, thereby allowing a bond to form.3. When the nuclei are infinitely far apart, there is no repulsion or attraction between them; thus, the potential energy approaches zero.4. Once the attractive forces are greater than the repulsive forces, the outer orbitals are able to overlap, thereby allowing a bond to form.5. As the internuclear distance decreases, the nuclear and electrostatic repulsions increase significantly, causing a steep…arrow_forward
- the maximum number of bond a carbon can make is?arrow_forwardWhat shape is a sulfur dioxide molecule?arrow_forwardWhich statement below correctly describes network covalent bonding, in most cases? A) Atoms of one or more metals bonded with covalent bonds in a lattice structure. B) Atoms of one or more nonmetal elements bonded with covalent bonds in a lattice structure. C) Atoms of one or more nonmetal elements bonded with covalent bonds into molecules that are weakly held by intermolecular forces. D) Atoms of a metal and nonmetal elements bonded with covalent bonds in a lattice structure. Note: Please select the write optionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning