
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
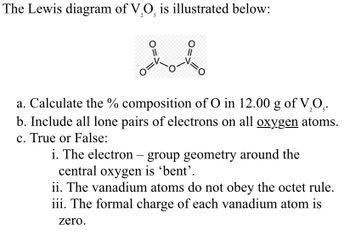
Transcribed Image Text:The Lewis diagram of V₂O is illustrated below:
O=
1=0
a. Calculate the % composition of O in 12.00 g of V₂O₂.
b. Include all lone pairs of electrons on all oxygen atoms.
c. True or False:
i. The electron - group geometry around the
central oxygen is 'bent'.
ii. The vanadium atoms do not obey the octet rule.
iii. The formal charge of each vanadium atom is
zero.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for carbon dioxide , CO2 . The concepts of formal charge and electronegativity can help us choose the structure that is the most significant representation.1. Assign formal charges to the elements in each of the structures below. A B C Formal ChargeO1CO2 2. The structure that contributes most significantly to the overall electronic structure of CO2 is (A,B, or C) .arrow_forwardPOST-LABORATORY QUESTIONS Due after Laboratory Experiment 1. Thiocyanate, SCN, has three possible structures a. Draw the resonance structures of thiocyanate, SCN- b. Assign the formal charge of each atom in each of the three resonance structures. On the basis of the formal charges and the electronegativities, identify the major resonance structure. с. Duour tho LOuris dot structure for each of the following molecules or ions. Determinearrow_forwardFill in the lone pairs on each atom to give every main group element except hydrogen an octet.arrow_forward
- 1. The heat of atomization is the amount of energy required to break up a molecule into its atoms. Use average bond energies in your textbook to determine the heat of atomization of C2H3CI. H CI X H Harrow_forward1. How many bonds must each of the following atoms have in order to have no formal charge? a. Carbon b. Hydrogen c. Oxygen d. Chlorine e. Nitrogen 2. Using the information from question #1, determine whether each of the following structures is possible or impossible? (Note that if no formal charges are shown, this implies that all formal charges are zero.) H H H H I MacBook Pro H Harrow_forwardDraw an Octet Rule Lewis Structure for: 1. ICl3 2. XeO3 3. B2Oarrow_forward
- How many non-bonding (lone) electron pairs are in the Lewis structure for NHCI2? O 5 6. 8. O 9 OOOarrow_forwardMINDTAP Formal Charge 01 Three inequivalent Lewis structures for carbon dioxide, CO₂, are shown below. Use the concepts of formal charge and electronegativity to choose the structure that is the best representation by answering the questions below. 1. Assign formal charges to the elements in each of the structures. C 0₂ :O-c=0: A Use the References to access important values if needed for this question. 2. The best Lewis structure for CO₂ is 0₁-C=0, B :0,=C-0₂: Q Search this cours Carrow_forward5. Write Lewis structures that obey the octet rule for each of the following: a) PHC1₂ c) Br₂CO b) ASH [3]arrow_forward
- The molecules below have ionic bonds. Name them. Most of them have covalent bonds also. Draw Lewis Structures of each, including resonance structures. NaCl (NH4),SO4 LİBH4 36 40. 37. NH.Cl 41. 38. Ba(OH)2 KC1O4 42. KC;H;O2 39.arrow_forward93. Draw the Lewis structure of SOF₄ (with minimized formal charges) and then determine if the molecule is polar or nonpolar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY