
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
5. thermodynamics
Transcribed Image Text:C
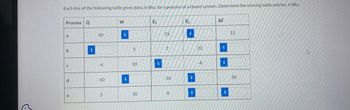
Each line of the following table gives data, in Btu, for a process of a closed system. Determine the missing table entries, in Btu.
Process Q
a
b
C
d
e
40
-4
-10
3
W
i
5
10
10
E₁
i
15
7
-10
8
E₂
i
22
-8
ΔΕ
i
i
i
15
30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. Compared to gasoline, hydrogen has (A) higher energy density per unit volume and per unit mass (B) higher energy density per unit volume but lower energy density per unit mass density per unit mass (C) lower energy density per unit volume but higher energy (D) lower energy density per unit volume and per unit mass 2. A new hydroelectric power plant proposed for the Mississippi River near St. Louis is advertised as "delivering 438,000 megawatt-hours of renewable electricity to the region annually". What is the average power output of the plant? (A) 50 MW (B) 1.2 GW (C) 438 GW (D) 438 MW 3. A car accelerates from 0 to 60 miles/hour in 6 seconds. What is a good estimate of the power output of the engine? (A) the mass of the car divided by 60 miles (B) the chemical energy of the gasoline consumed during the acceleration (C) the thermal energy produced by the engine during the acceleration (D) the final kinetic energy of the car divided by 6 secondsarrow_forward6 thermodynamicsarrow_forwardDifferentiate zeroth, first, and second law of thermodynamicsarrow_forward
- The molar heat capacities of substances varies with temperature. The general function for determining the molar heat capacity is given below; Cp = ( a + b T + c T 2 )R . In case of a gas where a = 3.245, b = 7.108 x10 ^-4 K ^-1 , and c = -4.06 x10^ -8 K ^-2 for temperatures in the range of 300 Kelvins to 1,500 Kelvins. What is the heat capacity (in Joules per Kelvin per mole) of this gas at 1,500 kelvins? NOTE: Express answer in THREE SIGNIFICANT FIGURES.arrow_forwardWhich of the following principles is the closest interpretation of the first law of thermodynamics? O Energy is continuously and indefinitely discharged more than it receives. O The mass within a closed control volume does not change. O The change of the total energy is equal to the rate if work performed. ON O All real processes tend toward increased entropy. O The net energy crossing the system boundary is the change in energy inside the system.arrow_forwardState the difference between extensive, intensive and specific properties of a thermodynamic system.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY